views
Anticipating a Covid-19 vaccine launch by the end of 2020, the world was hawkishly watching over this field’s development. To immense credit for science and human ingenuity, we did meet an almost impossible deadline. The pace of vaccine development was unprecedented, as was the uncertainty around the rapidly mutating novel coronavirus. Given quick roll-outs under emergency approvals universally, the scepticism and questions over vaccines were warranted. Both science and the governments are obligated to allay peoples’ apprehension around their health. This is due especially when opposing claims, having a free run on social media, confirm pre-existing biases. Disinformation tends to mask the scientific evidence if not debunked pre-emptively This article explores scientific evidence in an attempt to answer frequently asked questions and allay some concerns on Covid-19 vaccines.
Available vaccines:
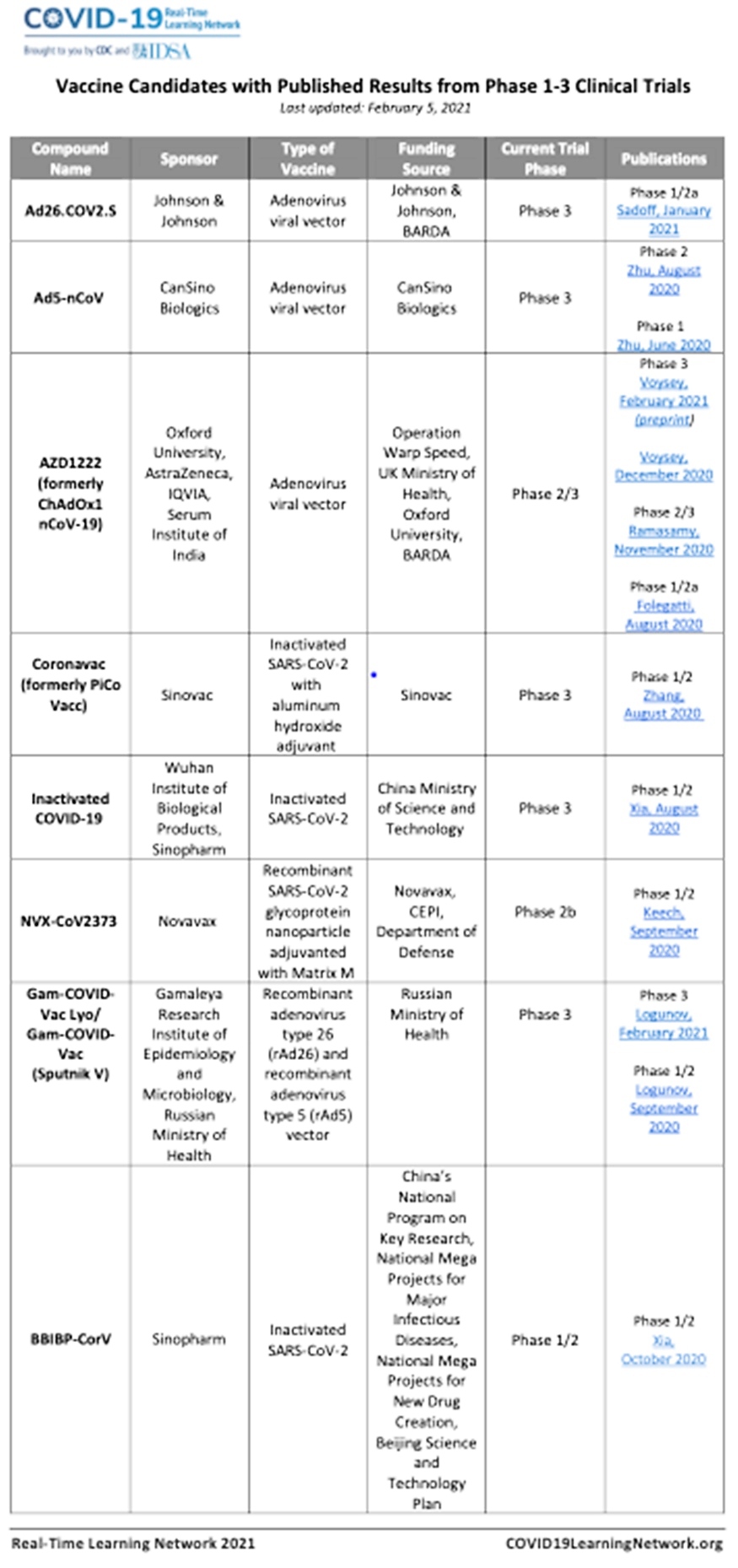
There are currently more than 60 Covid-19 vaccine candidates in clinical development and over 170 in pre-clinical development worldwide.
India has authorised the Oxford/AstraZeneca vaccine (Covishield), a recombinant vaccine based on Viral Vector Technology (common cold virus from chimpanzees is made to look like coronavirus, but cannot cause the disease), and an indigenously developed whole-virion inactivated coronavirus vaccine called Covaxin by Bharat Biotech. Both can be stored at +2 to +80C, allowing existing cold chains and transmission routes to be deployed quickly after roll-out on January 16, 2021. The regimen is two doses administered intramuscularly, four weeks apart. Serum Institute of India (SII) manufactures 50 million doses a month, and Bharat Biotech assures 700 million doses in 2021.
Phases one and two of Covaxin clinical trials are complete, and the third with 25,800 participants is underway. The first two trials show that the vaccine generates an adequate immune response, and the side effects reported are mild to moderate only. The results of the final trial are due by the end of February.
Trials for Covishield conducted in the UK, South Africa, and Brazil report high efficacy and safety.
Both Moderna and Pfizer vaccines, used in the EU and the USA, have done three phases of trials and proved highly efficacious and safe.
Other highly promising vaccines completing phase 3 trials and likely to be launched soon are from Johnson and Johnson, Novavax, and Gam-Covid-VAC (Sputnik V).
Most manufacturers have also started trials on children between 6 and 18 years of age, and a children’s vaccine is to be available soon. Vaccinating children is necessary for achieving herd immunity for an infectious disease.
Q. When will we get our shots?
The goal of the vaccination programme in India is not to achieve herd immunity but to prevent severe disease and deaths among those most at risk- HCWs (Health Care Workers), FLWs (Front Line Workers), those above 50 years of age, and those with morbidities which is 22% of the population (approx. 300 million). They shall be covered in the first phase (started January 16, 2021) within 7-8 months.
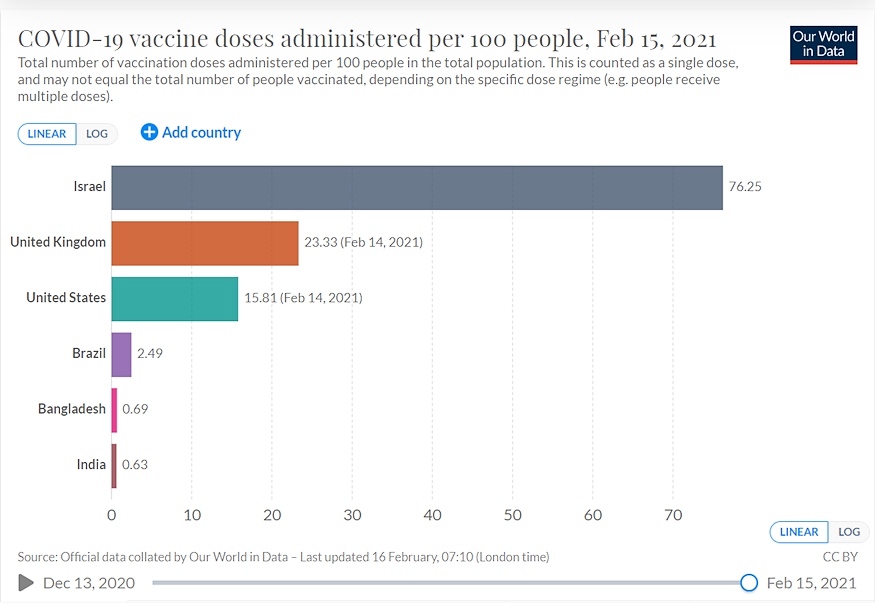
Given India’s population and availability constraints of vaccines, China and India, may not be sufficiently vaccinated until the end of 2022.
The Covid-19 vaccination coverage to healthcare and front line workers crossed 8.85 million doses on February 16, a month into the programme. Unless the coverage is sufficiently enhanced, the first phase may dilate to a couple of years or more at the current rate.

We may also have to contend with new variants of SARS-CoV-2, needing adjustments in type and dosage, delaying the coverage further.
Q. Mutations: Will the existing vaccines work on new variants of the virus?
After many months of relative stability, Covid-19 started mutating fast by the end of 2020 as the vaccines based on initial prototypes of SARS-CoV-2 began rolling out. Cases infected with three main reported variants from the UK, South Africa and Brazil have been found in India.
These variants spread faster and are likely to replace existing variants. The UK variant is deadlier for older age groups. The South Africa variant, though more evasive to existing vaccines, has not proved to be more lethal.
CDC, USA reports that antibodies generated through vaccination with currently authorised mRNA vaccines (Pfizer and Moderna) recognise these variants.
India’s key issue is whether vaccines authorised for use here will be able to protect against infection or disease from these new SARS-CoV-2 variants. Preliminary research results of the AstraZeneca vaccine (Covishield) showed 74% efficacy in the UK variant (B.1.1.7) but only 22% in the South Africa variant (B.1.351/ 501.V2). No data is available for Covaxin.
Fortunately, another protein-based Covid-19 vaccine, the Novavax nanoparticle vaccine, showed 89% efficacy in the UK and 49% efficacy in South Africa. More encouragingly, 85% protection against severe Covid-19 has been reported for the Johnson & Johnson vaccine in South Africa.
WHO has enhanced its genomic surveillance and information sharing on new variants while encouraging countries and manufacturers to be prepared to adjust to the SARS-CoV-2 viral evolution, including potentially providing future booster shots and adapted vaccines, if found to be scientifically necessary.
Q. Is it safe for pregnant and lactating mothers to take the vaccine?
India’s government has authorised use for pregnant and lactating health and front line workers who have a high risk of exposure, and the dangers of not taking the vaccine outweigh the benefits. WHO and CDC, USA also report that vaccine does not endanger pregnancy as per available research.
Q. Should people who have recovered from Covid receive the vaccine?
Experts say, yes.
Reinfection with Covid-19 is not reported before 90 days of recovery, and patients may defer vaccination for this period.
Patients treated with monoclonal antibodies or convalescent plasma should defer vaccination by 90 days as these treatments might inactivate the vaccines, making them less effective.
France’s health authority recommends single shots for people who have had Covid-19. Others stick to a two-dose regimen for these groups also.
Q. Reactogenicity: side effects – what to expect?
In both Pfizer and Moderna trials, no severe side effects were reported. AstraZeneca did note a few severe side effects, in just 3 cases, that were successfully treated. No deaths were reported among participants with any vaccine.
WHO Strategic Advisory Group of Experts on Immunization (SAGE) also declares Covishield safe to be used for people 18 years and above.
We can expect mild side effects like pain at the site of reaction and mild fever with all vaccines. Body aches, chills, and headaches may occur temporarily. These are the common side effects faced with most other vaccines, including the flu vaccine.
Q. How far apart must two doses be?
Immunogenicity increases with a longer interdose interval; WHO recommends a gap of 8 to 12 weeks between them.
Q. How long does the vaccine keep one protected?
We do not have data on how long the immunity will persist after two doses of any vaccine. Phase 1 trial of Moderna vaccine showed that antibodies stayed for four months. Dr. Paul E. Sax, MD, professor of Medicine at Harvard, informs that there is currently no specific recommendation for booster doses given the absence of information on how long the vaccines will be protective. WHO also says that the need for, and timing of, additional doses will be evaluated as further data accumulate.
Q. Is vaccination enough to stop the pandemic?
Vaccines would not be enough to get us over the pandemic soon as developing herd immunity would require 90% or more people of the world vaccinated (To achieve protection among 70% of people given the efficacy of vaccines that range from 66% for Johnson and Johnson to 95% for Pfizer). This being a borderless disease, ending the pandemic entails vaccinating all the countries on the planet together within a similar time frame. Given the logistics constraints, that does not seem likely. And at the current pace, it will take 7 years for 70-80% of the globe to get vaccinated.
The emergence of new strains makes the matter more complicated, and the goal of achieving herd immunity more protracted.
To reduce transmission to levels that Covid-19 spread is contained within localised pockets (it achieves endemicity like HIV and Tuberculosis), government advice on nonpharmaceutical interventions must continue to be followed by vaccinated individuals as well as those who have not yet been vaccinated.
We are also unsure whether vaccination prevents transmission of virus, like Measles vaccine, or only prevents the disease but not transmission, like the Diphtheria vaccine. Until we know for sure that vaccines prevent transmission, mask-wearing, social distancing, and hygiene will continue to be mandatory to prevent spread.
Q. How to get more people to accept and receive the vaccine?
What is of immediate concern for India is that just 4% of people who got vaccinated on day one of launch returned for their second dose. (Ministry of Health and Family Welfare, India on February 13)
The Government of India and WHO‘s communication strategies lay down a practical roadmap to inform the public about vaccine safety for protection against the disease. It is sine qua non to bring the economy back on track.
Professor K. “Vish” Viswanath, the Lee Kum Kee Professor of Health Communication at Harvard, informs that disinformation (deliberate spread of untruth with a motive to deceive vs. misinformation that false information without fact-checks spread without an intent to mislead) about vaccination is a massive challenge before governments and health organisations trying to increase vaccine uptake. Rampant social media mainstreams disinformation. Some platforms are self-censoring and have already started working on removing malcontent. Unfortunately, most are not accountable. Therefore, the governments need to maintain keen oversight while communicating the truth directly to citizens.
India has less of a problem convincing people for acceptance of vaccine given a lifelong association of citizens with a publicly delivered, most extensive vaccination program in the world that has seldom failed. Thus, the trust in the government is high. India’s government reports high anticipation and eagerness among the public to receive Covid-19 vaccine in contrast to the USA, where vaccine hesitancy is high, especially among more vulnerable minorities. The logistics, communication, and outreach efforts of the Government of India are worth emulating for countries who find it challenging to convince their citizens on the merits of Covid-19 vaccines and their essentiality in bringing our lives back to pre-Covid-19 normal.
The efforts of the state of Odisha in India in this regard are an excellent case in point. The health workers, whose backgrounds are concordant with local communities, share their experiences openly after receiving their shots. Highly credible sources like heads of state-level health institutes are also speaking to the public directly on various fora. Uptake and acceptance of vaccines are consequently higher, and Odisha has vaccinated 4,18,000 beneficiaries and aims to vaccinate 100% of the target (all HCWs and FLWs by the end of February, a cool six months in advance of the national target).
Q. How effectively has India leveraged its position as a global vaccine leader during the crisis?
India has pulled her supremacy as a leading vaccine manufacturer to great advantage, thus turning adversity into opportunity. More than 20 vaccines are being developed, and at least six more vaccines are likely to complete safety and efficacy trials in India soon. One is a nasal vaccine by Bharat Biotech. We have shipped vaccines to Bhutan, Maldives, Bangladesh, Nepal, Myanmar, the Seychelles, some as a gift as part of vaccine diplomacy, and others under commercial agreements. Sri Lanka, Afghanistan, Brazil, and Canada are other countries we plan to send vaccines to. The UK is auditing facilities at SII to grant approval of imports of Covishield to the UK, which shall also allow us to send vaccines to other countries that accept UK approvals for orders.
India is a significant supplier to WHO’s COVAX facility, a global initiative bringing together governments and manufacturers to ensure eventual Covid-19 vaccines to poorer countries.
We have come a long way from the time the pandemic struck. We now know enough about the modes of transmission and mechanism of action of virus, the forms the Covid-19 disease can take, what therapies work and what drugs don’t. And most impressive of all, we have developed safe and effective vaccines in an unprecedented time. However, the germ we are fighting against has not stopped evolving. If vaccine coverage is not scientific and optimal, it might facilitate newer and deadlier mutations in viruses that evade the immune response. In light of this danger, people must get vaccine shots in recommended time, and governments must not let up the effort of bringing fast as many people under coverage as possible. And remember, vaccines need the help of a full arsenal of non-pharmacological measures like masks and distance measures to defeat the pandemic.
Read all the Latest News, Breaking News and Coronavirus News here




















Comments
0 comment