views
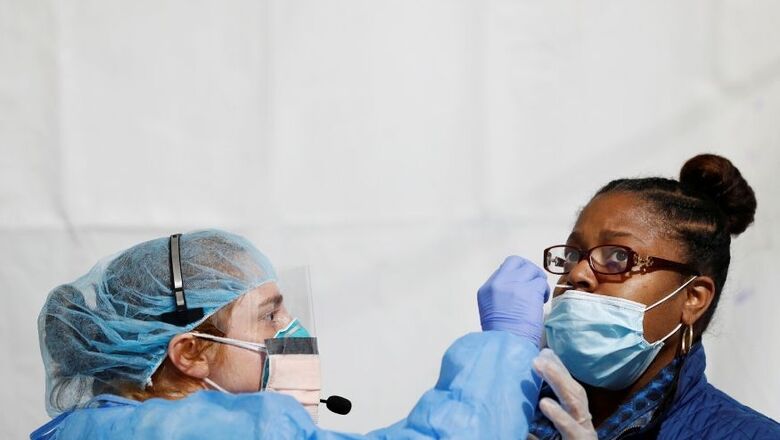
Scientists have developed a low-cost swab test that can diagnose COVID-19 infection in about 45 minutes, an advance that may help public health officials scrambling to cope with testing backlogs as the number of cases continues to climb.
The test named the "SARS-CoV-2 DETECTR" is easy to implement and to interpret, and requires no specialised equipment, said researchers from the University of California, San Francisco (UCSF) in the US.
This quality is likely to make the test, described in a paper published in the journal Nature Biotechnology, more widely available than the current crop of COVID-19 test kits, they said.
Though the new test has yet to receive formal approval for clinical use from the US Food and Drug Administration, the researchers are clinically validating the test in an effort to fast-track the approval process.
"The introduction and availability of CRISPR technology will accelerate deployment of the next generation of tests to diagnose COVID-19 infection," said Charles Chiu, a professor at UCSF, and co-lead developer of the new test.
The new SARS-CoV-2 DETECTR assay is among the first to use CRISPR gene-targeting technology to test for the presence of the novel coronavirus, the researchers said.
Since CRISPR can be modified to target any genetic sequence, the developers "programmed" the test to home in on two target regions in the genome of the novel coronavirus.
One of these sequences is common to all "SARS-like" coronaviruses, while the other is unique to SARS-CoV-2, which causes COVID-19, according to the researchers.
Testing for the presence of both sequences ensures that the new DETECTR tool can distinguish between SARS-CoV-2 and closely related viruses, they said.
Much like the diagnostic kits currently in use, the new test can detect the novel coronavirus in samples obtained from respiratory swabs, the researchers said.
While the widely used tests based on polymerase chain reaction (PCR) techniques take about four hours to produce a result from a respiratory sample, the new DETECTR test takes only 45 minutes, rapidly accelerating the pace of diagnosis, they said.
Researchers noted that another key advantage of the new DETECTR test is that it can be performed in virtually any lab, using off-the-shelf reagents and common equipment.
This stands in stark contrast to PCR-based tests, which require expensive, specialized equipment, limiting those tests to well-equipped diagnostic labs, they said.
The new DETECTR test, researchers said, is easy to interpret: much like a store-bought pregnancy test, dark lines that appear on test strips indicate the presence of viral genes.
They said it is very sensitive and can detect the presence of as few as 10 coronaviruses in a microlitre of fluid taken from a patient -- a volume many hundreds of times smaller than an average drop of water.
Though slightly less sensitive than existing PCR-based tests, which can detect as few as 3.2 copies of the virus per microlitre, the difference is unlikely to have a noticeable impact in diagnosis, as infected patients typically have much higher viral loads, the researchers said.
















Comments
0 comment