views
Spurred by the presence of China-made gelatin in Indian medicines, the nation’s top health regulatory body, Central Drugs Standard Control Organisation (CDSCO) is on the verge of tightening guidelines, urging a strict adherence to pharmaceutical-grade raw material, News18 has learnt.
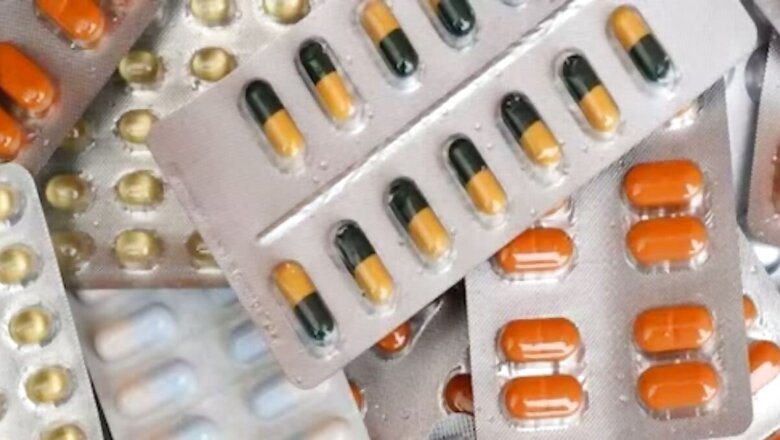
Industrial-grade gelatin, known to contain cancer-causing substances, is being used to manufacture medicinal capsules in India. Gel-like semi solid substance, generally used as covering of medicines, gelatin is derived from processed collagen obtained from animal skin, bones, cartilage and tendons.
The issue has raised concerns in terms of usage of cheap industrial grade products in medicinal products and triggered the government to consider bringing in the proposal for appropriate regulatory intervention to ensure that safe and pharma grade “inactive bulk drugs” are imported and used in drug making.
According to the minutes of the meeting of the latest Drugs Consultative Committee (DCC) by the CDSCO, accessed by News18, an advisory will be issued to all state licensing authorities (SLAs) to direct all manufacturers under their jurisdiction to use only pharma grade excipients to manufacture drug formulations. The DCC has also decided to constitute a committee to examine the matter and give a comprehensive report within three months.
The recommendations have been approved by the Drug Controller General of India (DCGI), head of CDSCO and have been sent to states and union territories for further action.
Gelatin is not the only inactive bill material that is imported. In fact, the minutes of the meeting noted that “during risk based inspections and investigations for international complaint (for exporting substandard cough syrups), it was observed that excipients like glycerin, propylene glycol etc. were imported from different countries as industrial grade and supplied by traders/not licensed under Drugs and Cosmetics Act and Rules thereunder to the pharmaceutical manufacturing units”.
The international complaints received from The Gambia, Uzbekistan, the United States, Marshall Islands and Micronesia raised doubts over the quality of Indian medicines after people in those countries suffered the consequences.
HOW DID GELATIN COME UNDER THE SCANNER?
The minutes show that a presentation was received by the government about technical or industrial grade gelatin imported from China being used for the manufacture of capsules — both soft and hard.
Further, the DCC noted: “Nagrik Upbhokta Margdarshak Manch has also reported that some of the capsule manufacturers are knowingly or unknowingly using industrial grade gelatine imported from China in the manufacturing of capsules intended for human consumption.”
According to the minutes, the DCC was informed that “there is an increasing evidence that low cost industrial or technical grade gelatin unfit for human consumption is being imported mainly from China, by unscrupulous traders and sold to soft capsule manufacturers who blend it with high quality gelatin made in India, in the manufacturing of capsules.”
It said that “technical gelatin is prohibited for human consumption due to the presence of certain toxic (carcinogenic) compounds of chromium etc as there is no regulation specifying maximum limit of chromium or other heavy metals or salmonella and requirement for drawl of samples for testing of industrial grade gelatin to ensure safety”.
The panel, as per the minutes, was informed that another representation was also received that India imports around 120,000 MT of industrial grade IPA (Isopropyl Alchohol) — an important solvent for pharmaceutical applications. However, usage of industrial grade IPA that does not fulfill pharmacopeia criteria is dangerous and hazardous to health.
The representative had informed the government that 170,000 MT of IPA consumed by the pharmaceutical industry and only about 15-17% is pharmacopeia grade.
“Industrial grade IPA lands in the pharma industry, apart from serving the other industries like coatings, cleaning etc. Imports mainly come from China, South Korea, Taiwan, Europe, and USA,” the document said.
THE DECISIONS
The DCC, as per the minutes, has decided that as per the Drugs and Cosmetics Act “only pharma grade excipients” shall be used and therefore proper enforcement needs to be done to tackle the issue.
To handle the issue, the drug controller general of India has informed state and regional offices to take action on DDC’s recommendations.
The DCGI has asked states to issue an advisory to direct all manufacturers under their jurisdiction to use only pharma grade excipients for manufacturing of drug formulations.



















Comments
0 comment