views
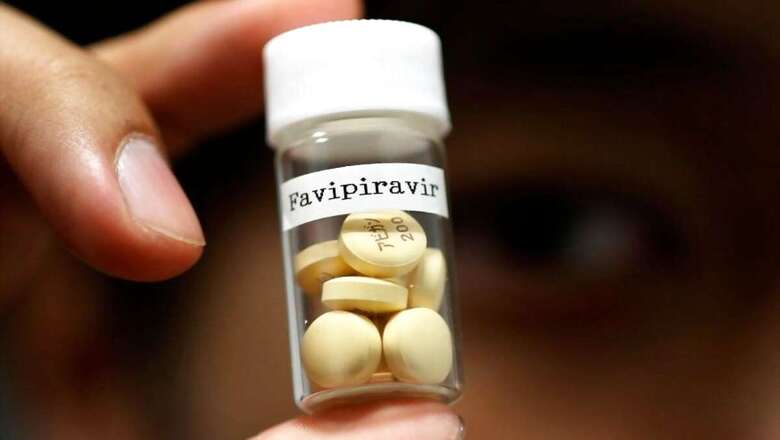
Drug firm FDC Ltd on Tuesday said it has launched two variants of the COVID-19 drug Favipiravir under the brand names PiFLU and Favenza. The Drug Controller General of India (DCGI) had earlier approved the use of Favipiravir, an off patent, oral antiviral drug that has been shown to quicken clinical recovery in COVID-19 patients with mild to moderate symptoms, FDC said in a statement.
"Early diagnosis and treatment will help in arresting the deteriorating condition of patients, and we will be working with the government and healthcare fraternity to make Favenza and PiFLU available across the country” FDC spokesperson Mayank Tikkha said. Both the products are currently available across the country, the company said.
The price per tablet is Rs 55 for both the variants, it added. Shares of FDC Ltd closed at Rs 317.50 per scrip on BSE, down 1.17 per cent over previous close.
.


















Comments
0 comment