
views
Attempting Mechanical Separation

Assess the different grain sizes of salt and sugar. At first glance, table salt and granulated sugar look very similar, including in size. The minute differences in the average grain sizes of the two, however, does provide an option for attempting separation. Regular table salt has an average grain size of 100 microns, or 0.1 mm. Note that other types of household salt, such as kosher or pickling salt, will have widely divergent average grain sizes. Regular granulated sugar has an average grain size of 500 microns (0.5 mm), or five times larger than table salt. Again, other sugars, like powdered or brown sugar, will have very different average sizes.
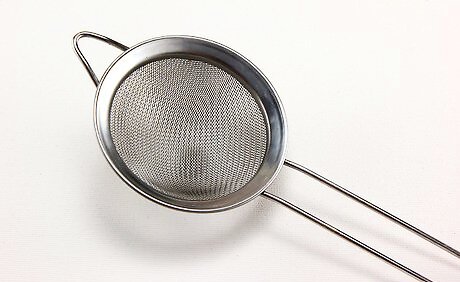
Acquire a sieve that is sized in between these grain sizes. Laboratory-style sieves (or strainers) are sized according to the spaces between the mesh. You want to find one that is large enough to let the salt through, but small enough to stop the sugar. Since salt is 100 microns across and sugar is 500, a 250 micron (0.25 mm) sieve would make a good in-between choice.

Get shaking. This method is a simple as it seems. Add small amounts of the salt-sugar mixture at a time to the sieve (with a bowl underneath), and shake, shimmy, and rattle it to slowly but surely send much of the salt through the mesh openings and into the bowl. Because this method relies on the difference in average grain sizes, it is never going to be entirely successful, There will be some smaller sugar grains that slip through and some larger salt grains that stay put, not to mention those that might stick together — at least until the point when you’re tired of sifting. Despite its limitations, however, sieving is a legitimate scientific means of separation. Just don’t expect to use the separated sugar in your coffee, unless you like a salty kick!
Dissolving and Evaporating the Mixture

Consider a simpler, safer alternative for a science experiment. If you are teaching or learning about separating materials and/or making solutions, think about using salt and sand as your mixed materials instead of salt and sugar. It is a bit easier, much safer, and equally interesting. Separating salt and sand involves adding warm water to the combination in order to dissolve the salt, straining out the sand by pouring the water mix through a fine sieve, then carefully boiling the water to leave the salt behind. It does not involve flammable liquids or potentially dangerous fumes. The safety issue is likely the main reason why it is hard to find lesson plans or legitimate scientific advice about how to separate salt and sugar. If you insist upon doing so, however, take every precaution. Do not try it at home unless you are well-versed in chemistry and have observed all safety measures. First and foremost, always have a working fire extinguisher nearby.

Add ethanol to your salt and sugar mixture. The larger the amount of salt and sugar, the more ethanol you will need to use. There must be enough alcohol that the sugar dissolves without being oversaturated. If at all possible, consider using only a small amount of salt and sugar or doing your separation in batches if you have a large amount. Ethanol is flammable and using too much will increase your fire risk.

Mix the solution with a spoon or a stir stick to dissolve the sugar. Once the mixture settles, the salt should be sitting at the bottom of the beaker. Granulated sugar is a substance containing carbon molecules that is soluble in alcohol and other organic solvents (such as acetone, for example). Table salt, however, is far less soluble in alcohol than it is in water because the former’s lesser polarity provides less attraction to salt’s sodium and chlorine ions.

Pour the alcohol solution through a very fine strainer, and into the new container. Your strainer should have gathered all the salt particles. Let the strainer dry and then pour the salt into a separate container. Remember that table salt has an average grain size of 100 microns, so you will need a strainer with smaller mesh openings than that. You may want to instead use a coffee filter placed inside the sieve.

Wait for the alcohol to evaporate, or create a steam bath. To make a steam bath, place a small saucepan filled one-quarter full with water on your heating element. Make sure you can set a glass bowl directly on top of the saucepan so that the bottom of the bowl does not contact the water in the saucepan. A steam bath is similar to a double boiler used in cooking.

Place the sugar and ethanol mixture in the open bowl on top of the steam bath. Turn on a fan or a hood and wear a mask to avoid breathing in the alcohol fumes. Only after placing the alcohol solution in the top bowl, heat up the water to a low boil on medium heat. The steam bath is designed to gently heat the solution because of the alcohol’s volatility. Other methods can cause a spark and light the alcohol. Do not let the alcohol solution come in contact with the heating element or any open flame. Stay away from the steam forming at the top of your open container of sugar and alcohol while it distills.

Continue the process until all the alcohol has evaporated. The sugar will be left in the open dish. Pour the sugar into a separate container. Exercise caution until and even beyond the conclusion of the experiment. Any remaining alcohol residue could be ignited by the still-hot heating element.














Comments
0 comment