views
X
Research source
Determining the number of neutrons in an atom is fairly simple and doesn’t even require any experimentation. To calculate the number of neutrons in a regular atom or an isotope, all you need to do is follow these instructions with a periodic table in hand.
Finding the Number of Neutrons in a Regular Atom
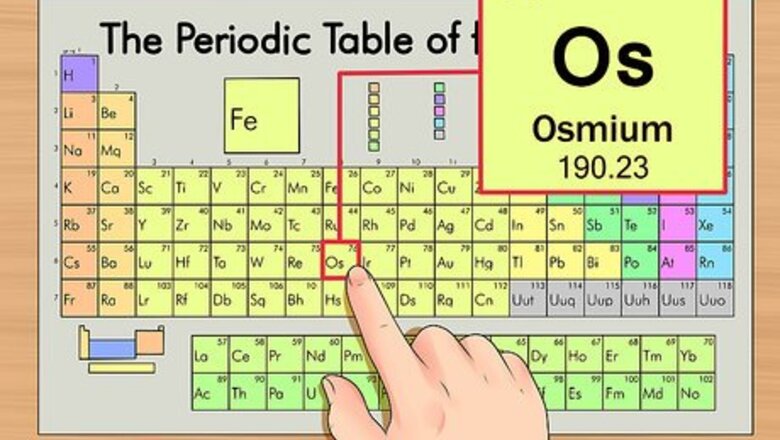
Locate the element on the periodic table. For this example, we’ll look at osmium (Os), which is in the sixth row down.
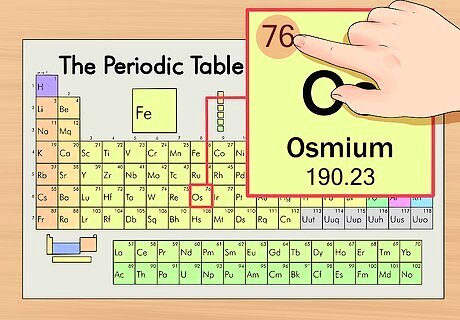
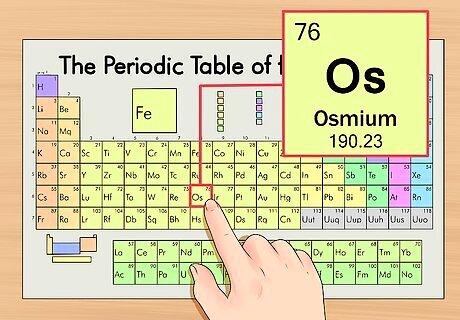
Find the element’s atomic number. This tends to be the most visible number pertaining to a given element and usually sits above the element symbol, either in the middle of the box or in the upper left corner. (On the chart we're using, in fact, no other numbers are listed.) The atomic number is the number of protons in a single atom of that element. Os is number 76, meaning one atom of osmium has 76 protons. The proton number never changes in an element; it's basically what makes that element that element.
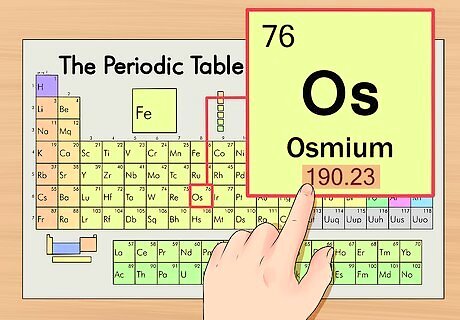
Find the element’s atomic weight. This number is usually found beneath the atomic symbol. Note that the chart in this example is based solely on atomic number and doesn’t list the atomic weight. This won’t always usually be the case. Osmium has an atomic weight of 190.23.
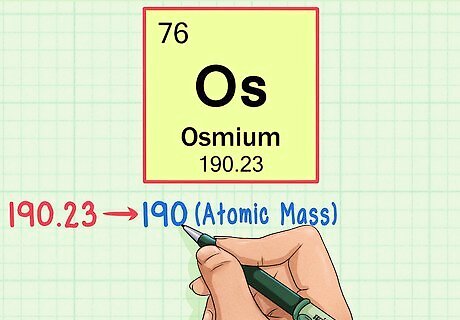
Round off the atomic weight to the nearest whole number to find the atomic mass. In our example, 190.23 would be rounded to 190, resulting in an atomic mass of 190 for osmium. The atomic weight is an average of the isotopes of the element, so that's why it's not usually a whole number.

Subtract the atomic number from the atomic mass. Since the vast majority of an atom’s mass is made up of its protons and neutrons, subtracting the number of protons (i.e. the atomic number) from the atomic mass will give you the calculated number of neutrons in the atom. The numbers after the decimal point represent the usually very small mass of the electrons in the atom. In our example, this is: 190 (atomic weight) – 76 (number of protons) = 114 (number of neutrons).
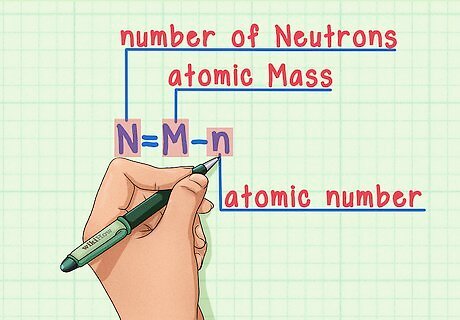
Remember the formula. To find the number of neutrons in the future, simply use this formula: N = M – n N = number of Neutrons M = atomic Mass n = atomic number
Finding the Number of Neutrons in an Isotope
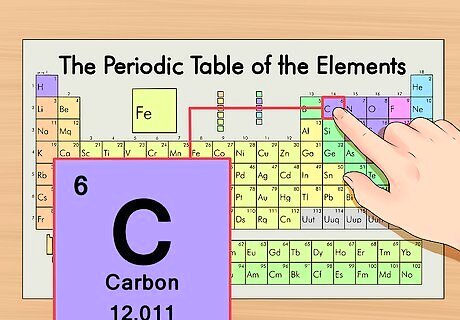
Locate the element on the periodic table. As an example, we’ll look at the carbon-14 isotope. Since the non-isotopic form of carbon-14 is simply carbon (C), find carbon on the periodic table (in the second row down).
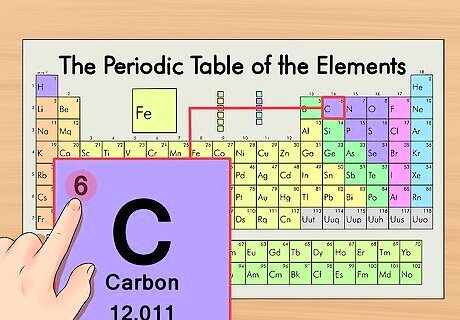
Find the element’s atomic number. This tends to be the most visible number pertaining to a given element and usually sits above the element symbol. (On our example chart, in fact, no other numbers are listed.) The atomic number is the number of protons in a single atom of that element. C is number 6, meaning one atom of carbon has 6 protons.
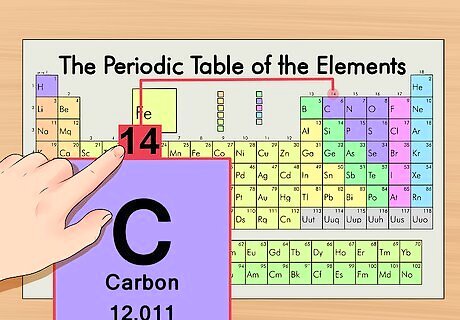
Find the atomic mass. This is incredibly easy with isotopes, as they are named according to their atomic mass. Carbon-14, for example, has an atomic mass of 14. Once you find the atomic mass of the isotope, the process is the same as it is for finding the number of neutrons in a regular atom.
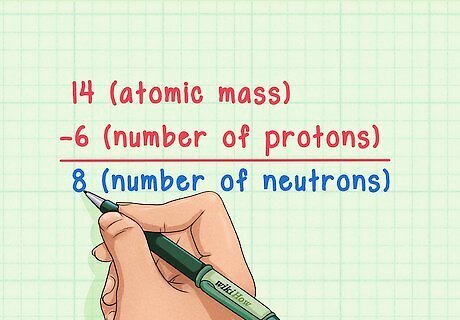
Subtract the atomic number from the atomic mass. Since the vast majority of an atom’s mass is found its protons and neutrons, subtracting the number of protons (i.e. the atomic number) from the atomic mass will give you the calculated number of neutrons in the atom. In our example, this is: 14 (atomic mass) – 6 (number of protons) = 8 (number of neutrons).
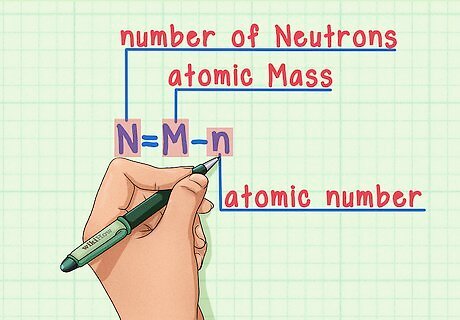
Remember the formula. To find the number of neutrons in the future, simply use this formula: N = M – n N = number of Neutrons M = atomic Mass n = atomic number



















Comments
0 comment