views
Testing the pH of Your Soil and Water

Get your soil professionally tested for the best, most accurate results. If you're serious about growing plants or making your soil more acidic for whatever reason, you'll know that professional sampling is more accurate than DIY home tests. It may not seem like it, but the difference between soil that is 5.5 and 6.5 on the pH scale is pretty big! If you are in the United States, contact the nearest county extension office. They will do a basic soil test, which includes measuring pH, for free or for a small fee.
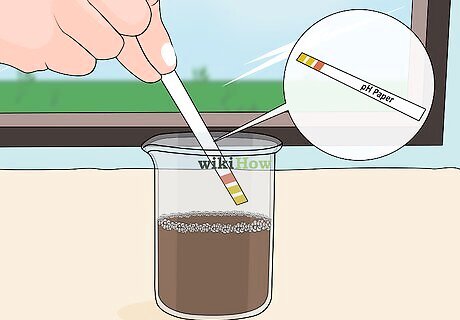
Try a DIY home pH test. If you're not into the idea of professional soil testing, you can easily test the pH of your soil at home, but understand that it won't be as accurate as a professional reading. There are several ways to get a pretty decent reading at home: Use paper strips to test pH. This method will only tell you whether your soil is predominantly acidic or basic, but it's a fun exercise that you can use with lots of different flowers, vegetables, and herbs. Use vinegar and baking soda to test pH. Another rudimentary way of testing acidic vs. basic, this method involves taking a cup of soil and dividing it into two containers. Add vinegar to one container and baking soda and water to the other, seeing which one fizzes. If it fizzes for vinegar, it's basic or alkaline; if it fizzes for baking soda, it's acidic. Buy a home testing kit. A home testing kit should be able to tell you your soil pH by giving you a number. This number is a more informative reading than the simple "this is acidic" or "this is basic" reading of over home methods.
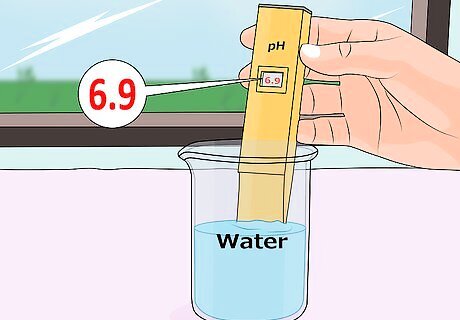
Be sure to test the pH of your water as well. The pH of groundwater that you may use to water your plants ranges from about 6.5 to 8.5, but is usually more on the alkaline side so that it doesn't corrode water pipes. If the water you use to water your plants is basic to begin with, and your soil is as well, know that you'll need a little extra "oomph" to produce the desired acidic effect for your plants. One way to get around this potential problem is to use pure, filtered water. Pure water has a pH of 7, which makes it almost completely neutral. Using pure, filtered water is effective, but it can become expensive quickly.

Know how to read the pH of whatever test you use. pH is a measure of how basic or acidic a substance is. This measure exists on a scale from 0 to 14, with 0 being very acidic (think battery acid) and 14 being very alkaline (think liquid drain cleaner). 7 is considered "neutral" on the pH scale. For example, if your soil reads 8.5 on the pH scale, it means that it's slightly basic. You'll need to add a little bit of acidic material to make the soil less basic. If your soil measures 6.5 on the pH scale, it means it's slightly acidic. If you want your soil to be even more acidic, you'll need to add additional acidic material. If you want to get into the nitty gritty, consider that pH is a logarithmic scale, meaning each number represents a 10-fold change. So a pH of 8 is 10 times more basic than a pH of 7, a pH of 8.5 is 15 times more basic, and so on.
Acidifying the Soil
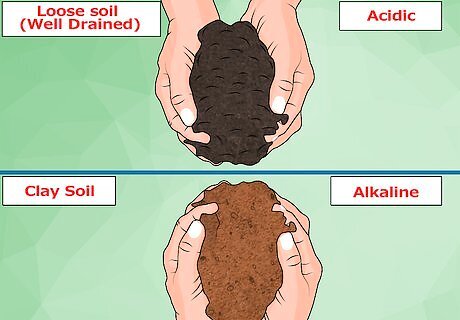
Identify your soil type. This is different than determining the pH of your soil, and it's a really important move. Your soil type will tell you which method of acidifying you should use. Soil that is already well-drained and relatively loose will make acidification much easier. This type of soil can benefit from large amounts of organic compounds that acidify the soil as they break down. Soil that is clumped with clay and seriously compacted will make acidification much tougher. Adding organic material to this type of soil will make it more alkaline, not less.
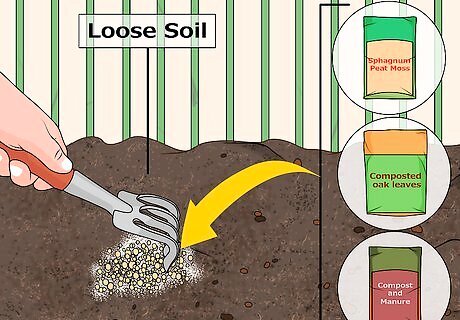
Add organic materials to well-drained, loose soils. In order to acidify these types of soils, organic materials are your best bet. Organic materials acidify the soil as they break down, but large amounts of them are needed to bring the pH down. Here are some nice organic materials you should consider using: Sphagnum peat moss Composted oak leaves Compost and manure
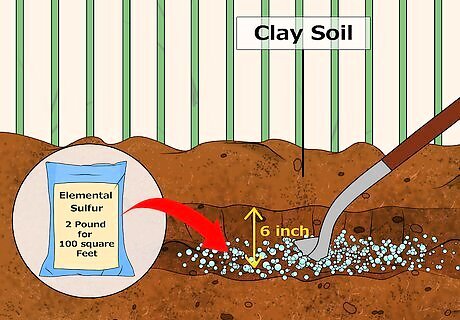
Add elemental sulfur to soil that is heavily compacted or which has lots of clay. As mentioned earlier, adding organic materials to highly-dense soil can make problems worse, as your soil retains more moisture, making it more alkaline. For this reason, adding elemental sulfur or iron sulfate is the most surefire way to acidify soil with heavy clay components to it. Elemental sulfur acidifies the soil as bacteria turns the elemental sulfur into sulfuric acid. It takes about 2 pounds of elemental sulfur per 100 square feet to reduce the pH of a soil that is 7 down to a pH of 4.5. Because elemental sulfur is slow to react, it's best to add it the year before planting in order to achieve the best results. Work the elemental sulfur into the soil, going as deep as 6 inches (15.2 cm).
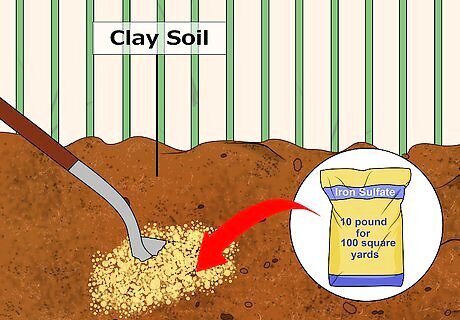
Add iron sulfate to soil that is heavily compacted or has lots of clay. Iron sulfate relies on a chemical reaction to create acidity. It is therefore less dependent on temperature conditions than elemental sulfur, which depends on bacteria to produce a biological reaction. It could take upwards of 10 pounds of iron sulfate for every 100 square feet of soil to reduce pH by one unit. If you are adding more than 10 pounds of iron sulfate for every 100 square feet of soil, then you will need to split it up into two applications, spaced one to two months apart. This will give the soil time to absorb the iron sulfate after application. Iron sulfates are lot faster-acting than elemental sulfurs. They can significantly reduce pH in a matter of three to four weeks, as opposed to several months. This gives them the added advantage of being usable the same season you decide on planting. Be careful when applying iron sulfates. They can cause rusty stains on clothes, sidewalks, and patios. It's best to separate any clothes you get iron sulfates on from other clothes — wash them separately to avoid any cross-contamination.

Use a fertilizer containing ammonia. In a lot of cases, all you need to do to acidify soil is use an ammonia-based fertilizer. Many of the fertilizers which are used for acid-loving plants contain ammonia sulfate or sulfur-coated urea. Calcium nitrate and potassium nitrate should not be used as fertilizers, even if they do contain ammonia. These fertilizers actually raise the pH of your soil.
Maintaining a Healthy pH for Your Plants

If flowers or plants already happen to be planted, use elemental sulfur. Because it is slow-acting, it's tough to make mistakes at recommended doses. Work it into moist soil as much as possible, without disrupting any root systems. Continue to monitor the pH of the soil as the months pass.
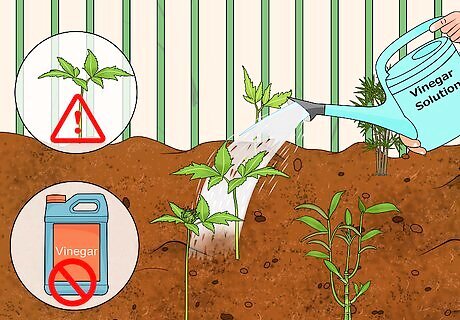
Resist the urge to add vinegar to your soil. Vinegar will lower the pH of the soil immediately, but in this case, it's not a great thing. The change happens too radically, disappears too quickly, and kills off beneficial soil organisms. Stay away from vinegar unless you're okay with the possibility of your plants dying.

Use cottonseed meal as an acidifying fertilizer over the course of the year. So you've already treated your soil with iron sulfates, for example, and you've just planted your blueberries. Keep the soil pH low by applying generous amounts of natural acidifying fertilizers such as cottonseed meal. Cottonseed meal, a by-product of cotton manufacturing, is especially great for acid-loving plants like azaleas, camellias, and rhododendrons.
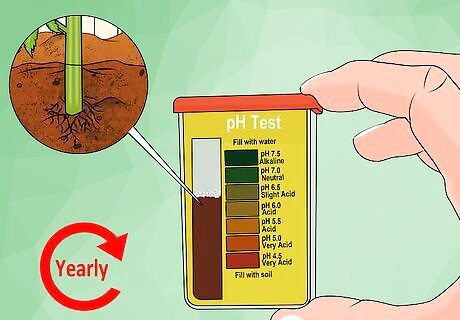
Check your pH at least every year. Check the pH of your soil near the base of your plants, adding fertilizers such as aluminum sulfate (especially for hydrangeas) without damaging the root system. For the best results, use a commercial pH testing kit or send off a sample of your soil to get professionally tested. Ornamental plants and vegetables will mostly prefer a slightly acidic environment of between 6.5 and 6.8. Hydrangeas, azaleas, rhododendrons, and blueberries will prefer a more acidic environment — between 5 and 5.5.
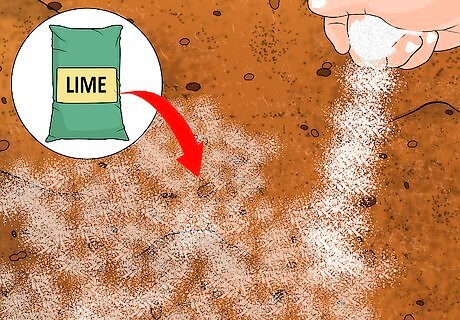
Raise the pH of your soil, if needed, by liming. In some cases, your efforts to acidify the soil will work too well and you'll be left with soil that is too acidic for your desired plant or vegetable. In these cases, you'll want to alkalize your soil with the addition of lime. Lime comes in three basic varieties — limestone, burned/quicklime, or hydrated lime — and how much to include will depend on the type of soil you have, as well as the variety of lime you choose to use. Inspect the packet for directions or talk to a horticulturist for more information.



















Comments
0 comment