views
Vaccine development is usually a long-drawn process with limited success. On average, it takes some 10 years to develop a new vaccine, while others take much longer. Of all vaccine candidates that enter the various phases of pre-clinical (in-vitro) and clinical (or human) trials, less than 10% succeed. Yet, the world witnessed an unprecedented race to develop various Covid-19 vaccines. More than 300 vaccine candidates have already entered various phases of pre-clinical and clinical trial stages. In less than a year of the SARS-CoV-2 (the virus that causes the infection) being reported, the first Covid-19 vaccine — the one by Pfizer-BioNTech — received emergency use authorisation (EUA) in the United Kingdom on December 2, 2020. Soon after, more vaccines were given EUA in many countries.
By early-May 2021, there were more than a dozen vaccines in use; one of the success factors has been the use & success of the new platforms to develop vaccines.
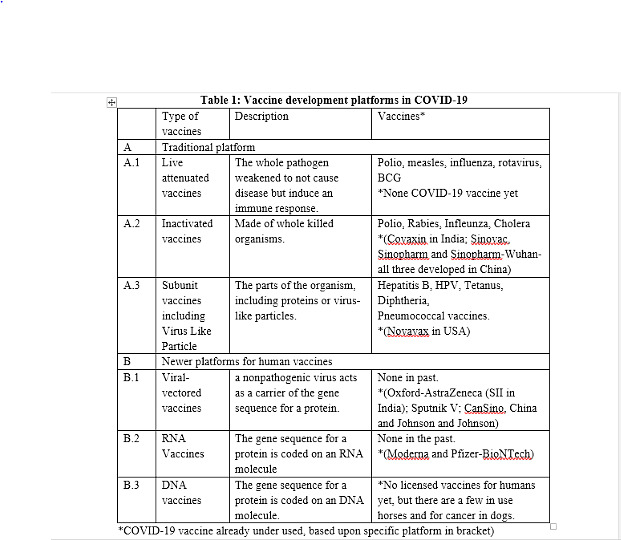
Traditionally, vaccines are developed as live-attenuated vaccines, inactivated or killed vaccines and sub-unit vaccines (Table 1). In response to the Covid-19 pandemic, vaccines have come up on viral-vectored and mRNA platforms, which had been under research and development for a few years. However, before Covid-19, no vaccine could be developed on these two platforms.
How vaccines work?
Vaccines train our immune system to fight a disease-causing agent, which has not yet affected a person. They prepare the body for future protection. Vaccines are known to have a component called antigen, which is usually a part of the pathogen against which the vaccine is being developed. The role of antigen, once inside the human body, is to activate the immune system in order to develop protective antibodies, without having any ability to cause the full-fledged disease. This way, once a person is fully vaccinated, he or she develops antibodies and remains protected. In majority of Covid-19 vaccines, the spike proteins in SARS-CoV-2 are optimal antigens, which are delivered in human body through various mechanisms.
Vaccines in India and the platforms used
There are two Covid-19 vaccines currently in use in India. Covishield (the vaccine by Oxford University-AstraZeneca) is based on the viral-vectored platform. The other vaccine, Covaxin (jointly developed by Bharat Biotech and the Indian Council of Medical Research), is an inactivated vaccine. A third vaccine, Sputnik V (of Russia’s Gamaleya Research Institute), which is already licensed and likely to be rolled out soon, is also based on the viral-vectored platform. Let’s understand both these platforms in detail.
Viral-vectored vaccines: A vector, in infectious disease biology, is what works as a vehicle to transport a disease-causing agent. For example, mosquitoes are the vector of malaria, a disease caused by a parasite called plasmodium. In viral-vectored vaccines, a virus is used to carry the target antigen gene into human cells. There are many such viral vectors, which have different advantages. The most widely known are the adenovirus vectors, which cause very mild colds or asymptomatic infections in humans. Covishield uses a chimpanzee adenovirus (AZD1222 or ChAdOx1), which carries the SARS-CoV-2 spike protein. The chimpanzee adenovirus has been used because humans will not have pre-existing antibodies to this adenovirus. Then there are human adenoviruses as well. However, the risk here is that previous colds or infections in an individual may leave them with antibodies to the human adenoviruses, which could interfere with the vaccine. For this reason, Sputnik V uses two human adenoviruses Ad5 and Ad26, aiming to reduce any interference with the second dose. The adenoviruses currently being used in Covid-19 vaccines are non-replicating, meaning that the virus given in the vaccine does not multiply. However, viral-vectored vaccines can also be made using viruses capable of multiplying, like measles and pox viruses. Some vaccines are using weakened influenza viruses as vectors so that a combination vaccine for both influenza and SARS-CoV-2 can be made. CanSino (developed by CanSino Biologics and Academy of Military studies) uses Ad5, and Johnson and Johnson’s vaccine uses Ad26.
Inactivated or killed vaccines: Pathogens (viruses or bacteria) that cannot multiply cannot cause disease. So inactivating a virus or bacteria, using chemicals like formalin, can convert them into a safe immunogen. Because inactivated viruses or bacteria do not multiply, we may need to use multiple doses of the vaccine and also give another substance to improve the immune response — this is called an adjuvant. The most common adjuvant is the alum but shark oil suspensions and a few others are also used. A number of vaccines developed in China, and Covaxin in India are on the inactivated platform.
In other parts of the world
There are a few more Covid-19 vaccines developed and licensed in other parts of the world (Table 1), but not yet available in India. However, in view of an Indian government decision, any Covid-19 vaccine licensed by regulatory authorities in the USA, the UK, Japan and the European Medical Agency, and those given EUA by the World Health Organization (WHO) would become eligible for licensing in India without completion of bridging trials. This will mean four additional vaccines — those by Moderna, Pfizer-BioNTech (both mRNA vaccines), Johnson and Johnson (viral-vectored vaccine) and SinoPharm (inactivated vaccine) — are eligible to apply for licensing in India. Let’s understand these platforms as well.
RNA vaccines: In this approach, the messenger RNA (mRNA) carrying the genetic sequence for the protein (spike protein of SARS-CoV-2) are enclosed usually in fatty nanoparticles. This resembles cell membranes and can deliver the RNA into the host cell, where the messenger RNA is treated like it belongs to that cell. Thereafter, the cell uses its protein-producing machinery to read the message and make spike protein, which is then released from the host cell and recognised by the immune system, triggering a response that results in both antibody production and cellular immunity. The first Covid-19 vaccine to reach clinical trial in March 2020 was mRNA-1273 from Moderna. The first licensed Covid-19 vaccine (anywhere in the world) was based upon this platform and developed by Pfizer-BioNTech. mRNA vaccines have a lot of advantages as a platform, because they can be made rapidly and have fewer long-term safety concerns; they cannot enter the nucleus and leave the bloodstream quickly.
Subunit vaccines, including protein or virus-like particles: A vaccine that consists of a whole or part of a protein is called a protein or subunit vaccine. The parts of the organism that are important to induce the immune response may be purified from the whole organism, or could be synthesized separately as just proteins. These could have a simple structure or a complex one, such as virus-like particles (VLP). Many subunit or protein vaccines are produced in bacterial or fungal cells, so that large amounts of protein can be made easily. Many companies are working on protein vaccines based on the spike protein of SARS-CoV-2. Some, depending on the size of the protein, will need an adjuvant. A related approach to protein vaccines is to make larger proteins that fold themselves to resemble whole virus-like particles, an approach which has been previously used for human papilloma virus (HPV) vaccines.
Other platforms under being looked at
Live-attenuated vaccines: This is a traditional platform on which a number of vaccines are already available. In this approach, the pathogen is weakened to make them non-pathogenic (ability to cause the full disease), but retain the immunogenicity (ability to mount immune response). In Covid-19, since the whole virus can cause full-fledged disease, the mutations are introduced to render the virus unable to cause disease, but have the ability to induce an immune response. Traditionally, the making of these mutations was left to chance by repeatedly growing the pathogens outside their hosts, but now new molecular approaches make it possible to make many mutations very precisely. A number of companies across the world, including two Indian companies (Serum Institute of India and Indian Immunologicals) are developing SARS-CoV-2 vaccines using this approach.
DNA vaccines: These vaccines resemble RNA vaccines in carrying the sequence for the gene codes for the protein that induces the right protective immune response. However, the process is a bit more complicated than mRNA vaccines. The DNA candidates must enter the cell and then the nucleus of a host cell to get the cell to make the messenger RNA. Thereafter, the messenger RNA will carry the sequence to where the protein is made. Getting the DNA into cells can be a challenge, and approaches include using electricity, or carrying the DNA on a plasmid to get it into immune cells in the skin or muscle. India’s Zydus Cadila’s vaccine is in process to develop vaccines based upon this approach, with the use of a DNA plasmid.
Conclusion
Science, research and vaccine development have been the reason of hope in the ongoing Covid-19 pandemic. Vaccine research and development globally has made a giant leap forward and the availability of many vaccines has raised the hope that we will win against the virus and the disease soon. In the end, when vaccines are licensed after clinical trial and review of data — no matter the platform on which they are developed — all serve a common purpose: to protect the vaccinated individual from the deadly disease.
Read all the Latest News, Breaking News and Coronavirus News here.




















Comments
0 comment