views
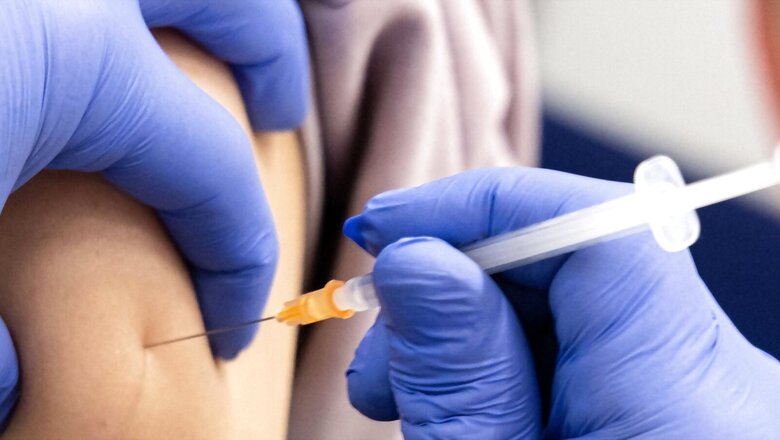
The Centre on Thursday announced the “Cervavac”, India’s first indigenously-developed vaccine for prevention of cervical cancer, will be launched in the next few months and will be available in the price range of Rs 200-Rs 400.
Cervical cancer ranks as the 2nd most prevalent cancers in India and accounts for nearly one-fourth of the world’s cervical cancer deaths despite being largely preventable.
As the announcement comes with much enthusiasm from the government and medical community, here’s why this development is so important. News18 explains:
Cervical Cancer a Widely Prevalent Disease
As per current estimates, every year, approximately 1.25 lakh women are diagnosed with cervical cancer, and over 75,000 die from the disease in India, and 83 per cent of invasive cervical cancers are attributed to HPVs 16 or 18 in India, and 70 per cent of cases worldwide.
Cervical cancer in India ranks as the second most frequent cancer among women between 15 and 44 years of age. This is why an affordable vaccine becomes of utmost importance to safeguard women’s health.
What Was the Vaccine’s Price Before?
Currently, two foreign-manufactured HPV vaccines are available in India: a quadrivalent vaccine (Gardasil Merck), which costs Rs 2,800 per dose, and a bivalent vaccine (Cervarix from GlaxoSmithKline), which costs Rs 3,299 per dose.
But according to Adar C. Poonawalla, CEO, Serum Institute of India, the first indigenously developed Quadrivalent Human Papillomavirus vaccine (qHPV) vaccine “Cervavac” will be launched in few months, and the vaccine will be available to the people in an affordable price range of Rs 200-400.
“It is likely to be priced in the Rs 200-400 range. But we will finalise it after discussing it with the government of India. Once our manufacturing starts, we will be able to talk more about it… I can assure everyone that it is going to be very affordable. It is going to be substantially lower than what is available today from manufacturers across the globe,” Poonawalla had said, addressing an event held on Thursday to announce the development of the vaccine.
Taking from Covid Lessons
Speaking at the event, Union Minister of Science and Technology Jitendra Singh had said the vaccine will be affordable and the government will ensure that it is accessible to the common man. Scientific completion implies that R&D activities pertaining to the vaccine are complete and now the next step of making them available to the public would take place.
Singh said Covid has raised awareness about preventive healthcare leading to the development of vaccines like the one against cervical cancer. “The schemes like Ayushman Bharat have made us think about preventive healthcare and we can now afford it. The Department of Biotechnology has taken a lead in the matter and are in collaborative mode,” he had said.
“Scientific efforts at times do not get the scale of recognition they deserve. So this event is to celebrate that scientific completion,” he had said.
Focus on Girls, But Vaccine Will Help Boys Too
Dr. Neerja Bhatla, professor (gynaecology and obstetrics), All India Institute of Medical Sciences (AIIMS) told the Hindustan Times that while the vaccine’s initial focus will be on women, particularly young girls, it will eventually be administered to boys to prevent a variety of other cancers.
“Because women are the most vulnerable group, priority will be given to them. Once that population has been vaccinated, the vaccine can be extended to young boys to prevent a variety of cancers, including anal and penile cancers. If even 70% of the female population is covered, there will be herd immunity and diseases can be controlled to a large extent,” Bhatla explained.
How Does the New Vaccine Work?
According to DBT officials, the new vaccine, like the hepatitis B vaccine, is based on VLP (virus-like particles) and is designed to provide protection by generating antibodies against the HPV virus’s L1 protein.
This will be especially beneficial to India’s nearly 50 million girls aged 9 to 14.
The Vision Behind the Vaccine
Announcing the scientific completion, Union Science & Technology Minister Jitendra Singh had said that this affordable and cost-effective vaccine marks an important day for the DBT and the BIRAC as it takes India a step closer to PM Narendra Modi’s vision of Atmanirbhar Bharat.
He noted that the most promising intervention for preventing cervical cancer is vaccination against human papillomavirus (HPV). It is estimated that HPV types 16 and 18 (HPV-16 and HPV-18) together contribute to approximately 70 per cent of all invasive cervical cancer cases worldwide.
Poonawalla, in his brief address, said that the wellbeing and protection of mother and child is the core philosophy of Serum Institute as only a healthy India can be a productive India.
Jitendra Singh pointed out that within a year of implementation, the Mission Covid Suraksha demonstrated major achievements such as development of the world’s first DNA vaccine for Covid-19 by Cadila Healthcare which received Emergency Use Authorisation on August 20, 2021, and supporting the development of the nation’s first mRNA Vaccine and intranasal vaccine candidate against Covid-19.
He said that “Cervavac” is an outcome of a partnership of the DBT and the BIRAC with the Bill and Melinda Gates Foundation, supported by Serum Institute of India for the indigenous development of quadrivalent vaccine through its partnership programme ‘Grand Challenges India’.
What’s the Plan to Disburse the Vaccine & How Effective Is It?
Poonawalla had said that a plan to make 200 million doses is in place and first the vaccine would be given in India and only after needs of the country are fulfilled it will be exported to other countries. Dr N Kalaiselvi, Director General, CSIR said this is the first stepping stone and research in the field and it will further continue.
According to the officials, the qHPV vaccine CERVAVAC has demonstrated robust antibody response that is nearly 1,000 times higher than the baseline against all targeted HPV types and in all dose and age groups. The Drugs Controller General of India (DCGI) had in July granted market authorisation to the Serum Institute to manufacture the vaccine against cervical cancer.
With inputs from agencies
Read all the Latest Explainers News and Breaking News here

















Comments
0 comment