views
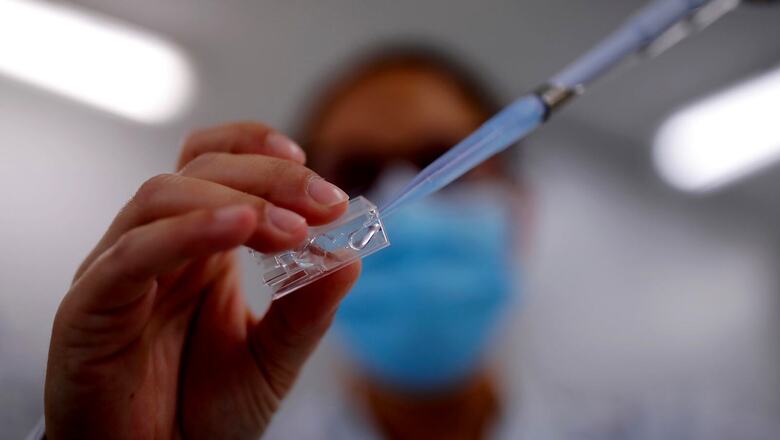
Bharat Biotech’s first-of-its-kind needle-free intranasal vaccine for Covid-19 has received approval from the Central Drugs Standard Control Organisation (CDSCO) under restricted use in an emergency situation for ages 18 and above, in India, for heterologous booster doses.
In heterologous boosting, a person administered a different vaccine from the one that was used for the primary dose series.
Bharat Biotech has said its Covid-19 intranasal vaccine, BBV154 or Incovacc, has proven to be safe, well-tolerated, and immunogenic in subjects in controlled clinical trials phase III. The vaccine candidate was evaluated earlier in phase I and II clinical trials with successful results.
Bharat Biotech chairman and managing director Krishna Ella said, “iNCOVACC, is an intranasal vaccine for the primary 2-dose schedule, and heterologous booster dose. This is a great achievement for us and the global scientific community to enable nasal administration of Covid vaccines. Despite the lack of demand for Covid vaccines, we continued product development in intranasal vaccines to ensure that we are well-prepared with platform technologies for future infectious diseases. iNCOVACC has been designed for efficient distribution, easy and pain-free administration. We have also initiated development of variant-specific vaccines for Covid for future preparedness.”
Rajesh S Gokhale, Secretary, DBT, and Chairperson, BIRAC, said, “The DCGI’s approval of Bharat Biotech’s intranasal vaccine iNCOVACC (BBV154) to be used as a heterologous booster dose against currently available Covid-19 vaccines is a moment of great pride for our country. This move will further strengthen our collective fight against the pandemic and broaden vaccine coverage.”
Ella had recently said that the firm completed clinical trials of the nasal vaccine with about 4,000 volunteers and there is no single instance of side effect or adverse reaction reported so far.
Origin Story
Bharat Biotech’s nasal vaccine uses a chimpanzee cold virus to deliver a harmless copy of the coronavirus spike protein to the lining of the nose. It has been specifically formulated to allow intranasal delivery and has been developed in partnership with Washington University St Louis, which had designed and developed the recombinant adenoviral vectored constructs and evaluated them in preclinical studies for efficacy.
Product development related to pre-clinical safety evaluation, large-scale manufacturing scale-up, formulation, and delivery device development, including human clinical trials, was conducted by Bharat Biotech. The Centre partially funded product development and clinical trials through the Department of Biotechnology’s, Covid Suraksha programme.
The DCGI had earlier given its nod to conduct clinical trials for Bharat Biotech’s intranasal vaccine as a booster dose. The drug regulator had also permitted the firm to conduct a phase 3 clinical trial to compare the immunogenicity and safety of BBV154 (intranasal) with Covaxin.
Two separate and simultaneous clinical trials were conducted to evaluate BBV154 as a primary dose (2-dose) schedule and a heterologous booster dose for subjects who have previously received two doses of the two commonly administered Covid vaccines in India.
How are Nasal Vaccines Different?
It’s hard for a shot in the arm to form lots of virus-fighting antibodies inside the nose where the coronavirus latches on. But a nasal vaccine might offer a new strategy to prevent infections that disrupt people’s everyday lives even if they’re mild.
An article published in Scientific American in March last year had urged developing nasal spray vaccines because they have an immediate effect on the virus in an infected person’s mucus. There they trigger production of an antibody known as immunoglobulin A, which can block infection. “This overwhelming response, called sterilising immunity, reduces the chance that people will pass on the virus,” said the article.
Researcher Nathalie Mielcarek, who is working with the Lille Pasteur Institute to develop a nasal spray vaccine against whooping cough, says stimulating immunity directly in the nose “lowers the risk of infecting other people”.
“From there you have less of the virus infecting the lungs and so fewer severe cases since the viral load is lower,” she had added.
Advantages of Nasal Vaccine
India’s inoculation drive so far has been dominated by Covishield, a domestically produced version of the AstraZeneca Covid-19 shot by Serum Institute of India, and Bharat Biotech’s inactivated vaccine Covaxin, both administered through injections.
But the nasal vaccine could be a game-changer in the quest to quell the pandemic. According to Bharat Biotech, the intranasal vaccine has the following advantages:
- The nasal route has excellent potential for vaccination due to the organized immune systems of the nasal mucosa
- Non-invasive, needle-free
- Ease of administration since it does not require trained healthcare workers
- Elimination of needle-associated risks like injuries and infections
- High compliance since it suits children and adults
- Scalable manufacturing and ability to meet global demand
An article published by Gavi the Vaccine Alliance has noted other advantages, including the fact that the sprays don’t need refrigeration and don’t need to be administered by health professionals. “People would be able to self-administer them at home,” the article says, adding “they are likely to be more popular for the millions of people who don’t like needles”.
Read all the Latest Explainers here














Comments
0 comment