views
A high-level panel of experts set up by the Narendra Modi government is meeting pharmaceutical industry officials this week to discuss ethical marketing practices, News18 has learnt.
On Friday, November 4, the third meeting of the committee is slated to take place to consider various issues pertaining to marketing practices by pharma firms.
On September 21, News18 first reported that the Modi government has formed a five-member panel to curb the bribing of doctors by pharmaceutical companies.
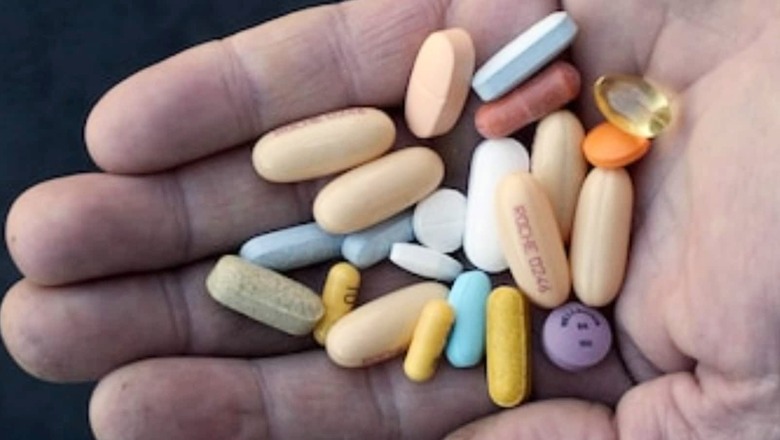
The controversy of the alleged bribing of doctors by the makers of Dolo, Micro Labs, brought the debate over the pharma-doctor nexus into the spotlight once again.
Prompted by the row, the central government formed a high-level panel to review the marketing practices of drugmakers and examine the requirement for a legally enforceable mechanism.
The panel is engaged in reviewing the existing code of conduct, the Uniform Code of Pharmaceutical Marketing Practices (UCPMP), which was rolled out in 2015 as a voluntary set of rules to be adopted by all the major pharmaceutical associations.
The panel is led by Dr VK Paul, a member (health) at NITI Aayog, as its chairman. The four other members are S Aparna, secretary of the department of pharmaceuticals, Rajesh Bhushan, secretary, ministry of health and family welfare, Nitin Gupta, chairman, Central Board of Direct Taxes (CBDT), and a joint secretary (policy) from the department of pharmaceuticals.
Under the same panel, now the industry representatives have been sent an invite for the discussion.
In the meeting, as part of stakeholder consultations, the committee desired to have the views of the Organisation of Pharmaceutical Producers of India (OPPI), the Indian Pharmaceutical Alliance (IPA), and the Indian Drug Manufacturers Association (IDMA) — the three lobbies representing domestic as well as foreign drugmakers in India.
In the invite sent to these associations, the panel has advised that they may consider making a presentation on their views.
“They have also kept a bar that these three associations may be represented by not more than three representatives each,” said a government official privy to the development. “The panel has to submit the recommendations to the government by December. This could be a significant meeting after which the members can finally start making a draft of recommendations.”
Read all the Latest India News here

















Comments
0 comment