views
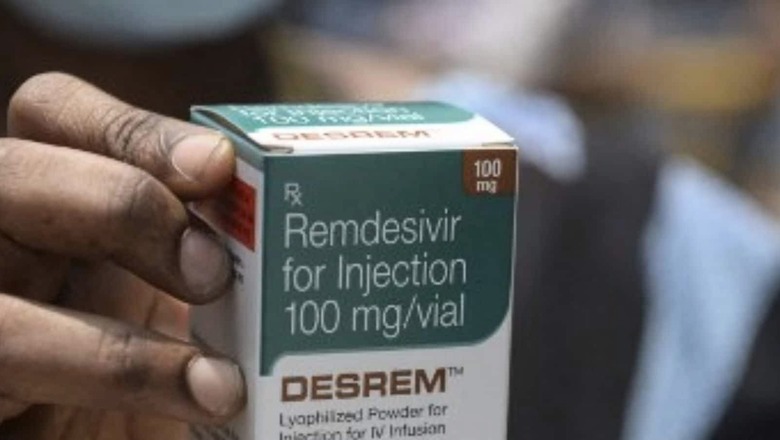
Why none of the pharmaceutical public sector units were not granted a licence to produce Remdesivir when the common man was facing “hardships” in procuring the anti-Covid drug during the second wave, the parliamentary standing committee on chemicals and fertilizers has made an observation in its report.
The committee also noted that during the second wave of the Covid-19 pandemic, the “shortage of this medicine caused severe hardships to the people across the country”.
Remdesivir, a patented drug, is manufactured in India by seven Indian pharmaceutical companies under voluntary licences granted by a US-based originator firm, Gilead Life Sciences. This drug has been included in the National Treatment Protocol of Covid-19 as an optional drug.
The panel further said the “affordable Covid-19 medicines and medical devices is the need of the hour during the unprecedented pandemic situation when the common man on the street is suffering either due to non-availability of Remdesivir or if available, an exorbitant price is charged making it difficult for the poor people to afford the medical treatment”.
“However, the committee failed to understand that none of the Pharma Public Sector Undertakings under the Department of Pharmaceuticals have been granted voluntary licence to manufacture Remdesivir and other Covid essential drugs for public health supply.”
It further said that it feels that equal opportunity should also be extended to these pharma PSUs who have developed trust, quality and cost effectiveness in their pharma products over a long period of time.
The committee, therefore, recommended that the Department of Pharmaceuticals — under the Union Ministry of Chemicals and Fertilizers — initiate steps to explore the possibilities of manufacturing of Covid essential drugs by PSUs under it.
Scientific Studies to Check Remdesivir’s Efficacy
The panel further recommended that scientific studies should be conducted to check the efficacy of Remdesivir.
“Since Remdesivir has been included in the National Treatment Protocol as an optional drug, the committee recommends that scientific studies should be conducted on the effectiveness of medicines like Remdesivir which have been included in national treatment protocol as optional medicines in curing critical Covid patients.”
Based on the studies, it said, steps should be taken by the ministry of health to remove those drugs which are not necessary for inclusion in the protocol.
How Production of Remdesivir was Increased?
The Department of Pharmaceuticals had informed the panel that “in order to substantially augment the production of Remdesivir, the Drugs Controller General (India) granted expeditious approval to 40 new manufacturing sites of the licensed manufacturers of Remdesivir.”
“This has led to increase in a number of Remdesivir manufacturing sites from 22 in mid-April 2021 to 62 at present,” the panel said.
The domestic production capacity of Remdesivir increased from around 38 lakh vials per month in last April to around 122 lakh vials per month last June.
It informed the panel that after the second wave, the demand for Remdesivir has come down considerably, and the demand-supply gap has reversed whereby supply is much more than the demand. Now, the states and Union Territories have been issued “guidelines for buffer stock management of Covid-19 drugs” and advised to procure and maintain additional stocks of Remdesivir and other drugs for preparedness to deal with any future requirements.
Read all the Latest News India and Breaking News here
















Comments
0 comment