views
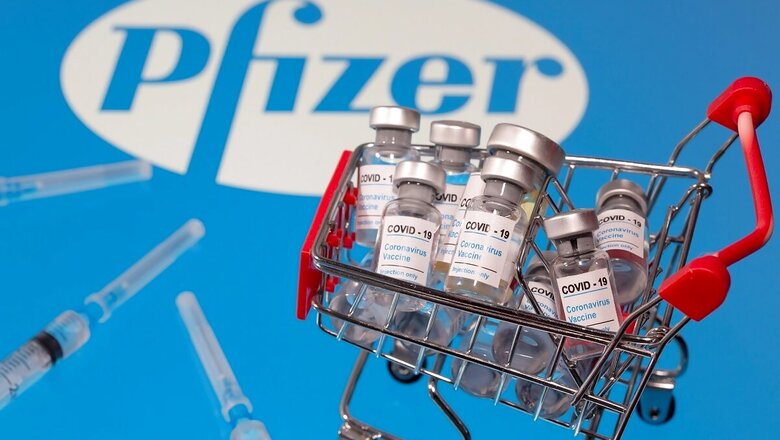
From insistence on seeking legal protection to wavering off clinical trials, Pfizer’s application to the Drugs Controller General of India (DCGI), seeking permission for Emergency Use Authorisation of its Covid-19 vaccine, has way too many ifs and buts.
It is expected that the subject expert committee constituted by the DCGI will meet this week to work out the modalities of Pfizer’s proposal. Pfizer and BioNtech’s mRNA vaccine, the first mRNA vaccine to get an approval in the history of medical science, has shown efficacy levels of over 90%. It applied for EUA to the DCGI on December 4. The pharmaceutical giant has got approvals in the UK and Bahrain.
INSISTENCE ON INDEMNITY
The UK has allowed emergency use authorisation to Pfizer, while deciding to give the company legal protection. This essentially means that the UK government will take the responsibility of treating any serious adverse outcome from the Pfizer vaccine. The DHSC (Department of Health and Social Care) UK has confirmed they would add this vaccine to the list of vaccines covered by Damages and Payments Act, which means that the government will pay a fixed amount for anyone who develops permanent disability as a result of the vaccination.
But is India, a developing country, going to be in a position to give legal protection of this nature to Pfizer? This has become a major hurdle in the path to giving a clearance to Pfizer with the government not keen on bearing this burden. “We are aware of demands by vaccine manufacturers for indemnity like in the case of occurrence of serious adverse events post-licensure. Where does the government stand on such demands? These are open questions,” says Malini Aisola, Co-convenor, All India Drug Action Network (AIDAN).
DOING AWAY WITH CLINICAL TRIALS
Pfizer also wants to skip bridging studies in India. Is this not unusual? No, say experts. “Vaccines that have already been through large-scale phase 3 clinical trials (involving tens of thousands of people) need not necessarily undergo the same trials in every country that wants to use that particular vaccine,” says Dr Rajeev Jayadevan, former president, Indian Medical Association, Cochin.
“This will not only delay the proceedings, but conducting such trials will become difficult, as other vaccines will already be out by then,” he says.
Experts say in such a setting, conducting a placebo-controlled trial will no longer be possible. The reason is that one cannot justify giving placebo anymore if a vaccine is already being recommended by the government. “This is a catch 22 situation in medical ethics,” he adds.
But the government so far has not allowed bridging studies to be skipped. Not in the case of Sputnik V, the Russian vaccine undergoing phase II, III trials in India, not even AstraZeneca, called COVISHIELD here, which is being trialled in the Indian population now.
“The Indian government has up till now maintained a principled stand that vaccines will be granted approval in India only after data is collected through local/bridging studies. It remains to be seen if the government relaxes its stand in respect of local trials not only for Pfizer but other potential applicants,” says Aisola.
NO DATA AVAILABLE
Data on vaccines so far has only been limited to company releases. “While there is no reason to disbelieve such press releases, doctors and scientists will invariably want to first look at peer reviewed papers published in reputed journals. For example, scientific publications will have subgroup analysis, through which any potential problems with the vaccine can be identified,” says Dr Jayadevan.
What one needs to understand from the data is how effective these vaccines are in vulnerable groups in whom the mortality and chance for severe complications is substantially higher. Hence, proving that there is efficacy only among a healthy young adult population will not be of much value here.
Such problems will not be obvious when you look at the overall numbers. Side effects from vaccines must be looked at differently from side-effects to other medicines. Therefore, any adverse outcome must be taken extremely seriously, more than any other category of medication.
STORAGE AND COSTS
The twin dilemmas before India; the vaccine is steeply priced at $39. It is an mRNA vaccine and carries the expense of novelty.
Because it is so expensive, the chances of it going down the drain if not stored right is a worrying proposition. While ensuring a longer shelf life of 6 months will require storage temperatures of -78 degrees, Pfizer says the vaccine can be stored at the regular 2-8 degrees and will have a shelf life of 30 days, with re-icing done every 5 days. Maybe an option for urban India, but certainly not an option for the interiors.
Read all the Latest News, Breaking News and Coronavirus News here


















Comments
0 comment