views
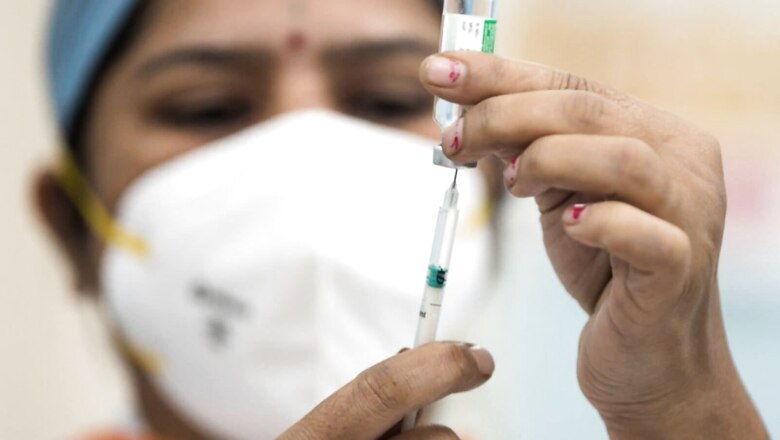
India is set to begin phase-2 clinical trials of the world’s first single-dose pneumococcal vaccine for adults, manufactured by Biological E, News18.com has learnt.
Pneumococcal vaccine – which is generally given in 4 shots to children and in at least 2 shots to adults – protects against several types of bacteria that can cause pneumococcal disease including bacteraemia, bacterial pneumonia, and meningitis.
Sponsored by Hyderabad-based Biological E, the phase one study of the investigational vaccine candidate was launched this year to evaluate safety, tolerability, reactogenicity, and immunogenicity of a single intramuscular dose in healthy adults in the 18-45 age group.
In the first phase, a total of 60 subjects were enrolled and the study has been conducted at only one site in India. Phase two trials, which are likely to involve 250 participants are expected to start in July.
The total duration of the phase 1 study was 35 days (7 days for screening and 28 days for follow-up) for each subject.
“Biological E’s 24-valent PCV is expected to provide serotype protection of greater than 85% in India,” according to the trial protocol synopsis submitted to the country’s drug regulatory agency, CDSCO, accessed by News18.com.
According to the 12-page document, there are more than 90 strains (serotypes) of pneumococcal bacteria and the first pneumococcal conjugate vaccine (PCV) PCV7 protects against seven of them.
Streptococcus pneumoniae causes paediatric invasive bacterial disease and is estimated to account for approximately 1 million deaths per year worldwide among children younger than 5 years of age. This is greater than the number of deaths from any infectious disease, such as HIV infection, malaria or tuberculosis.
So how is the vaccine different from the existing ones?
Apart from being a single-shot vaccine, the Biological E’s candidate “pneumococcal polysaccharide conjugate vaccine (24Valent) without preservative” aims to improve the global serotype coverage for enhanced protection by developing a 24 valent PCV (PCV24)”.
The candidate includes the most prevalent serotypes in India.
The serotype composition of this vaccine includes the prevalent serotypes isolated in GAVI eligible countries and are considered essential by GAVI in the new pneumococcal polysaccharide conjugate vaccines.
?Thus, it becomes extremely important to evaluate the circulating serotypes in a particular country and then select a suitable vaccine to ensure maximum efficacy and cost‐effectiveness,? said the protocol synopsis.
Based on data from various studies published between 1999 and 2013, the most prevalent serotypes currently in India are 14, 5, 1, 19F, 6B, 6A, 19A, 23F, 22F, 3, 9V, 18C, 4, 7F, 33F, and 17.1, the company mentioned in the document.
?This is evident from a recent study from India, which indicated that 54% of the pneumococcal isolates belonged to PCV‐10 and 73% to PCV‐13. Accordingly, a 24-valent PCV (PCV24) has been developed by Biological E that contains polysaccharides from the 13 serotypes included in PCV13 and eleven additional serotypes… specific to India,? it said.
Read all the Latest India News here

















Comments
0 comment