views
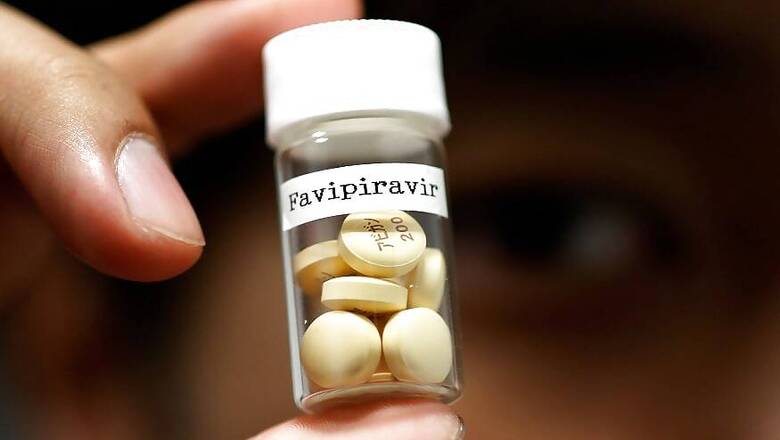
India's Glenmark Pharmaceuticals Ltd said on Wednesday its version of anti-flu drug favipiravir showed promise in a late-stage study of 150 patients with mild to moderate coronavirus infection.
Data showed that patients receiving FabiFlu shook off the virus about 29 per cent faster that those receiving standard supportive care.
About 70 per cent of the patients being treated with the drug achieved "clinical cure" by the fourth day of the study, compared with about 45 per cent in the standard care group, the company said in a statement.
Clinical cure is defined as a physician's assessment of the normalization of clinical signs of COVID-19 such as temperature, oxygen saturation, respiratory rate and cough.
FabiFlu was also well tolerated with no serious adverse events or deaths in the treatment arm, the company said, adding that it plans to submit the study data for peer review in the coming weeks.
Glenmark last month received Indian regulatory approval to make and sell FabiFlu for restricted emergency use in patients with mild-to-moderate COVID-19 symptoms, and last week cut the price of the drug to 75 rupees per tablet.


















Comments
0 comment