views
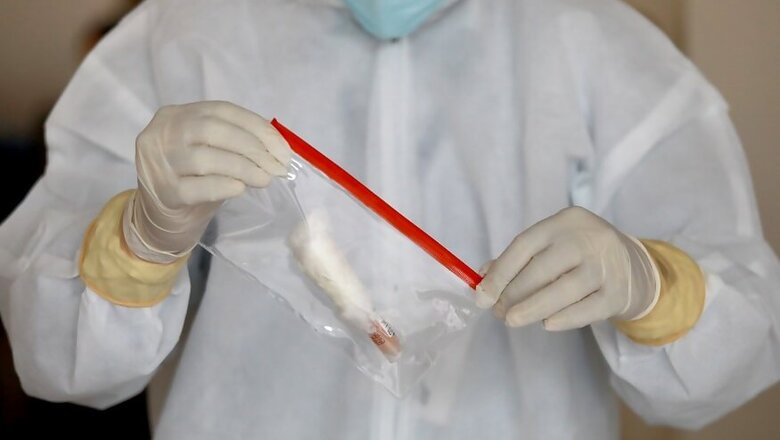
Indigenously developed ELISA testing kits for COVID-19 have been found to be sensitive and specific for the detection of novel coronavirus antibodies in human serum samples and can be used for determining infection exposure among the general population, a study said.
The kit may be used to detect exposed immune-protected individuals, stated the study conducted by scientists of the Indian Council of Medical Research (ICMR) along with other collaborators and the result published in the Indian Journal of Medical Research (IJMR).
ELISA testing kit was found to be 92.37 per cent sensitive, 97.9 per cent specific, robust and reproducible. The positive and negative predictive values were 94.44 and 98.14 per cent respectively, it stated.
ICMR, the apex health research body, has been conducting a survey to assess the proportion of population exposed to novel coronavirus, including asymptomatic individuals as a part of which ELISA-based antibody tests would be used for data collection.
Researchers highlighted that for surveillance of the emerging and re-emerging viruses, serological assays have been widely recommended. Several IgM/IgG ELISA kits for COVID-19 are in varied stages of development.
However, validated and approved SARS-CoV-2 serological assays are lacking for case detection and are not included in WHO's laboratory testing guidelines for COVID-19.
Due to non-availability of an indigenous, approved and cost-effective kit, an in-house ELISA was developed and validated for the detection of anti-SARS-CoV-2 human IgG antibodies, the study said.
A total of 513 blood samples were tested by the ELISA for COVID-19.
"Our findings suggested that this indigenous anti-SARS-CoV-2 human IgG-ELISA was sensitive and specific for the detection of IgG antibodies among individuals who have been exposed to coronavirus infection," the study highlighted.
It can be used for ascertaining the seroprevalence against SARS-CoV-2 in a population and for epidemiological studies, it said.
"ELISA will be useful in screening healthcare workers, industry workers among others. The use of whole-cell antigen instead of recombinant nucleocapsid antigen or spike protein antigen provided a broad sensitivity to the assay. However, the assay performance needs to be assessed in a large cohort of related human/zoonotic coronaviruses," the study added.
Such kits can be produced at low cost, are easy-to-use and affordable in resource-limited settings.

















Comments
0 comment