views
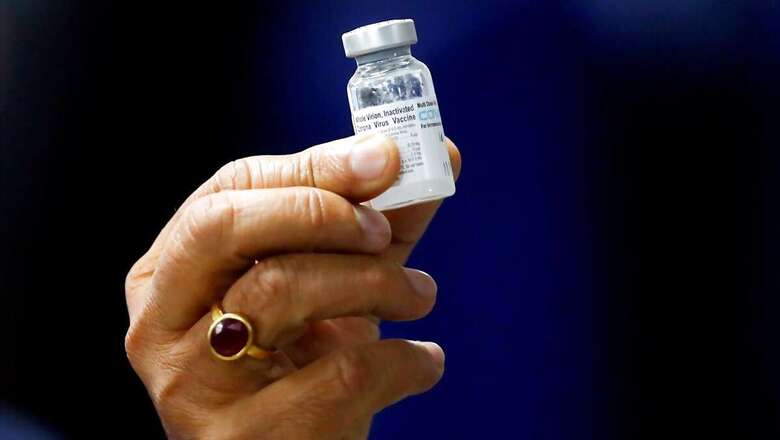
Hyderabad-based pharmaceutical firm Bharat Biotech on Monday announced all documents required for Covid-19 vaccine Covaxin in the emergency use list (EUL) has been submitted to the World Health Organisation (WHO) and the review process is ongoing, adding that the firm is expected to receive authorisation at the earliest.
“All documents required for Emergency Use Listing (EUL) of Covaxin have been submitted to WHO as of 9th July. The review process has now commenced with the expectation that we will receive EUL from the WHO at the earliest,” a statement by Chairman and Managing Director of Bharat Biotech, Dr Krishna Ella said.
COVAXIN® EUL pic.twitter.com/dCilH7KbSk— BharatBiotech (@BharatBiotech) July 12, 2021
The statement came days after WHO chief scientist Soumya Swaminathan said a decision regarding Covaxin’s emergency use listing will be announced within four to six weeks. Swaminathan had said on Saturday that Bharat Biotech was in the process of uploading its entire data on the health body’s portal.
According to WHO guidelines, EUL is a procedure to streamline the process by which new or unlicensed products can be used during public health emergencies.
“There is a process to be followed for EUL and pre-qualification of vaccines under which a company has to complete phase 3 trials and submit the whole data to the regulatory department of WHO which is examined by an expert advisory group,” Swaminathan said.
“The completeness of the data, which includes safety and efficacy and also the manufacturing quality, standard is provided. So, I expect that Bharat Biotech has already submitted data and in four to six weeks there will be a decision on its inclusion,” Swaminathan added.
The WHO has approved vaccines by Pfizer/BioNTech, Astrazeneca-SK Bio/Serum Institute of India, AstraZeneca EU, Janssen, Moderna and Sinopharm for emergency use.
Read all the Latest News, Breaking News and Coronavirus News here.














Comments
0 comment