views
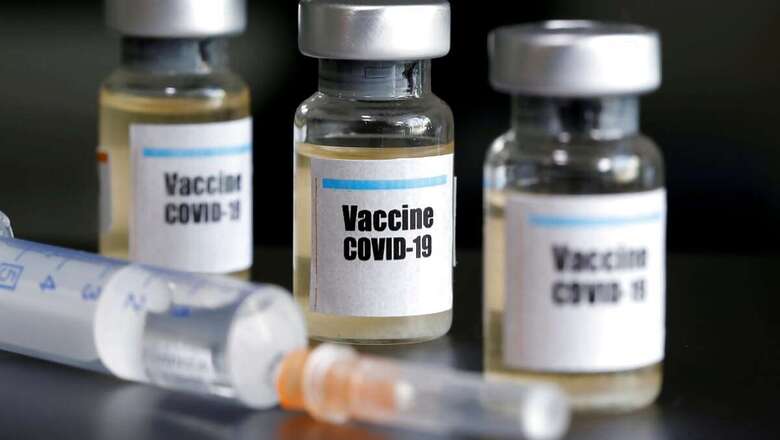
As the government’s national expert group decides on modalities that are key to the administration of a potential vaccine against coronavirus, experts and former top bureaucrats said that the size of the challenge is formidable. Globally, there are over 40 vaccines under various stages of trials whereas, in India, three vaccine candidates are undergoing clinical human trials. Two of them, developed by Bharat Biotech International Limited and Zydus Cadila, are being directly supported by the Indian government.
Keshav Desiraju, who served as Secretary, Ministry of Health and Family Welfare for a year between 2013 and 2014 said that administration of the Covid-19 vaccine for over a billion people will be the mother of all planning and logistic exercises. Desiraju said that the biggest difference between past vaccination campaigns, ongoing immunization programmes and the one against coronavirus will be the sheer scale of the job at hand. “Campaigns to eradicate polio or universal immunization involved reaching out to a targeted population. But the vaccination against coronavirus will have to reach out to almost the entire population. Now, that will be a mother of all planning and logistic exercises,” the retired bureaucrat said.
Desiraju said that the government will have to draw on all the previous major campaigns such as those against polio and HIV AIDS. He added that even as Serum Institute of India and Bharat Biotech are competent enough to manufacture vaccines, a lot of work will go into the processes such as storage, manufacture of devices needed to deliver the vaccine and last-mile delivery. Desiraju gave the example of cold-chains as one of the key logistical infrastructure issues that need to be addressed.
“Cold-chains are important because the vaccine has to retain its potency and efficacy. They cannot be stored at room temperature. The ground level workers such as ASHA (Accredited Social Health Activists) need ice-boxes and uninterrupted supply of electricity at places where vaccines are stored before last-mile delivery. Our vaccination programmes have been a success in the past due to the diligence of healthcare workers and sound logistics makes their job easier,” Desiraju said.
The many stages in vaccine administration and vaccination, especially in the case of a Covid-19 vaccine, begins from selecting a vaccine that will work. Last month, the Indian government constituted a national expert group on vaccine administration. This group has been tasked with the job of deciding on vaccine selection, procurement, delivery, prioritizing populations that would need first shots of the vaccine, sorting of logistics and inventory and planning for the funding of all these tasks.
“There is a digital platform that is being enhanced to track on a real-time basis, the vaccine movement from procurement to storage to administration and last-mile delivery. Another intervention will be made to develop online training modules for ground workers as skilled manpower will be required to vaccinate people, to report adverse events post-vaccination,” said Rajesh Bhushan, Secretary, Ministry of Health and Family Welfare.
“A sub-group within the national expert groups is seized of issues such as logistics, syringes and associated equipment,” he added.
India is part of GAVI, the public-private vaccine alliance that facilitates immunization and the Covax facility that has been formed to support research and development of Covid-19 vaccines, for negotiation of prices. The initial aim is to provide 2 billion doses by the end of 2021 to countries affiliated to the facility. More importantly, the facility is crucial for low and middle-income countries, including India, for getting equitable and affordable access to vaccines against Covid-19. India is eligible to receive vaccines from this facility and has pledged $15 mn to GAVI.
independent analysis of vaccine trial data
Eminent vaccine expert D Gagandeep Kang of the Christian Medical College, Vellore, said among many issues pertaining to vaccine administration, India needs to address two important issues relating to the independent review of data generated during vaccine trials and on what systems would be used to deliver it.
Kang cited the example of ‘Operation Warp Speed’, the public-private partnership of the Trump administration to facilitate, develop and distribute Covid-19 vaccines. “In this operation, the US government is providing individually negotiated funding to vaccine companies but it is insisting that those vaccine companies need to have aligned protocols and their samples and data need to be reviewed by independent labs and independent statisticians decided by the US government,” Dr Kang said.
This ensures a head-to-head comparison of the different vaccine candidates and comparability of data and of biological readouts, Dr Kang said.
“The Indian government is not doing anything like that at the moment. It should be thinking about this issue. For instance, if you have a definition of a clinical outcome that is severe disease versus another example in which clinical outcome is a mild disease; can you compare the two vaccines?” Kang said.
As far as delivery of vaccines is concerned, Kang said the government should consider at this stage what structure will be used to reach people; the existing immunization programme or a completely new one that perhaps uses Aadhaar, voter rolls or platforms? “Whatever we build should be new and rather not be repurposed from the existing system to prevent damage to it. We have already seen that ASHA workers are doing everything under the sun and that affected childbirths,” Kang added.
Read all the Latest News and Breaking News here
















Comments
0 comment