views
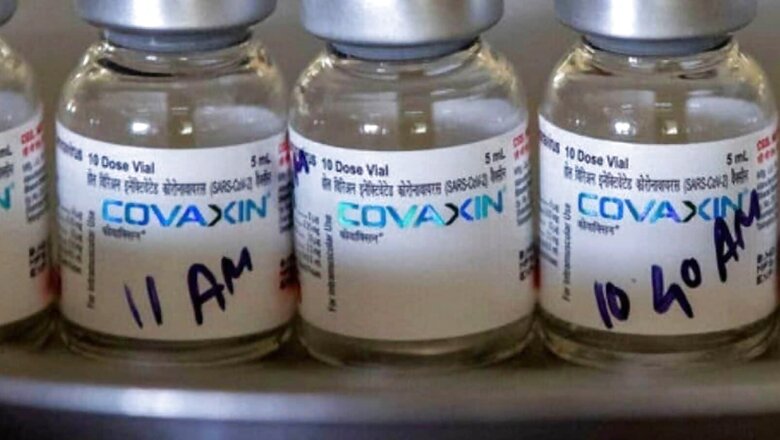
In the wake of concerns regarding quality of some batches of Covaxin from its Bangalore plant, Bharat Biotech on Thursday said it has uncompromising policy on safety and quality. National Immunization Technical Advisory Group (NTAGI) chief Dr NK Arora, on Tuesday, had said in response to short supply of Covaxin that the rejection of some batches in the initial days led to delay in vaccine production.
The pharma company confirmed that all batches of Covaxin, which is India’s only indigenous vaccine that has been rolled out so far, are manufactured and released only from their facilities at Genome Valley Hyderabad, which are fully audited and approved by regulatory authorities.
“Every batch of Covaxin is subjected to more than 200 quality control tests at our facilities, followed by submission samples to Central Drugs Laboratory (CDL) Government of India,” said Bharat Biotech and added that the batches are released commercially only based on approval and release by CDL.
The company cautioned against vaccine hesitancy by saying that that fake news and false and misleading narratives result in unintended consequences of creating panic in our population.
“The test batches of that plant weren’t satisfactory and got rejected which led to delay in ramping up production, but now the batches have been approved by the competent authority and will be available for public consumption,” Arora was quoted as saying by news agency ANI.
However, the statement said that the batches that are being produced in other facilities such as sites at Malur, Karnataka, and Ankleshwar, Gujarat will only be available from September.
Bharat Biotech appealed to media organisations and influencers “to use caution, restraint, detailed analysis in their reportage and their external communication.”
Covaxin showed 77.8% efficacy in phase 3 trials, the results of which were submitted in June 2021. The company’s phase 3 trial efficacy data was approved by the Centre’s Subject Expert Committee (SEC).
Read all the Latest News, Breaking News and Coronavirus News here.


















Comments
0 comment