views
Setting up the Equipment
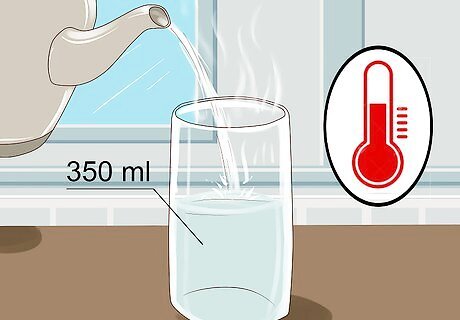
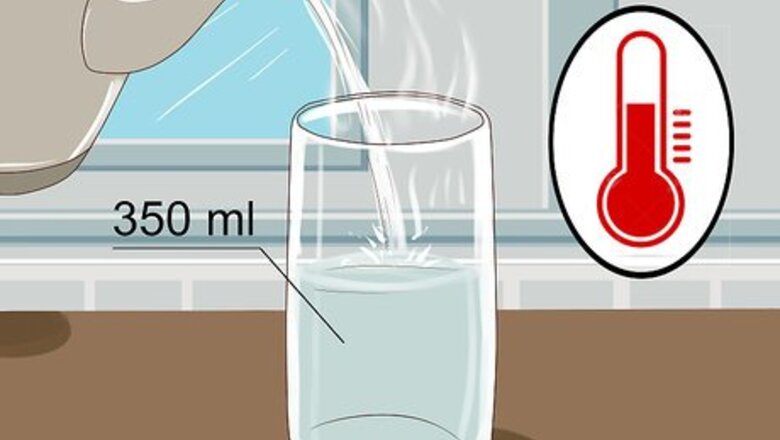
Fill a 350 ml (12 fl oz) glass with warm water. You don’t need to fill it all the way to the top, so leave a little bit of room. If the water is warm, it will conduct the electricity more effectively but it will also still work just fine with cold water. You can use either water from out of the tap or bottled water, it doesn't matter which. Warmer water has a lower viscosity and allows ions that conduct electricity to move more freely.
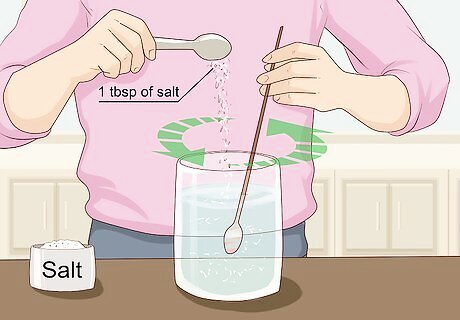
Dissolve 1 tbsp (17 g) of table salt in your water. All you need to do is pour it in and then stir it around a little bit to help it dissolve. This turns it into a saline solution. Sodium chloride (which is table salt) is an electrolyte which helps aid the conductivity of the water, as water by itself isn’t particularly conductive. By making the water more conductive, the current from the battery flows through more easily which results in the water being split into hydrogen and oxygen more effectively. You can also using baking soda (NaHCO3) instead of table salt. This will not produce chlorine gas.
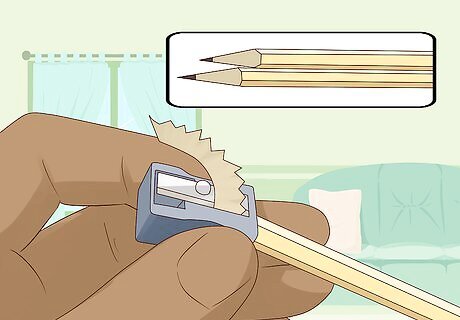
Sharpen both ends of 2 #2 pencils so the graphite is exposed. Make sure to remove the eraser at the top of the pencil. You need to sharpen the pencils enough that the graphite is fully exposed at both ends. These graphite shafts that are encased within the pencil are your electrodes and will conduct the electricity that comes from the battery. The graphite works really well as it doesn’t dissolve or get damaged in the water while you conduct the experiment. To increase the rate of electrolysis, make the graphite rough with the help of a blade or sandpaper. Consider using bare pencil leads from mechanical pencils to vastly increase the surface area. But handle them carefully as they are brittle.
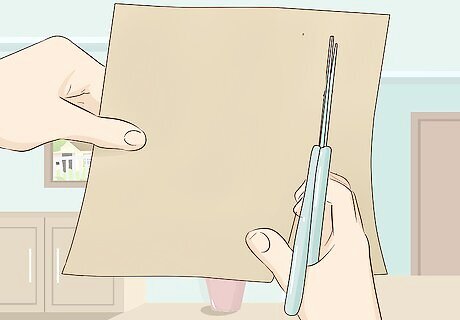
Cut a square piece of cardboard that is big enough to cover the glass. Use cardboard that is thick enough to maintain its structural integrity when it has some holes punched through it. Try cutting a square portion out of a shoebox or some other kind of thick box. The purpose of the cardboard is to suspend the pencil graphite in the water without allowing it to touch the walls of the glass. Because cardboard has no metallic properties to it, it can sit on top of your glass without affecting the outcome of the experiment.
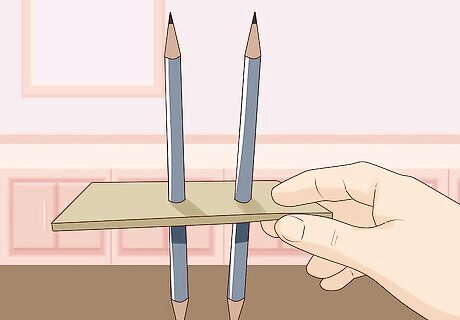
Punch 2 holes in the cardboard using the 2 pencils. Use the actual pencils to do this as you need to snugly fit the pencils into the holes so the pencils don't move around or slip. If the graphite touches the walls or the base of the glass, it will interrupt the experiment.
Conducting Your Experiment
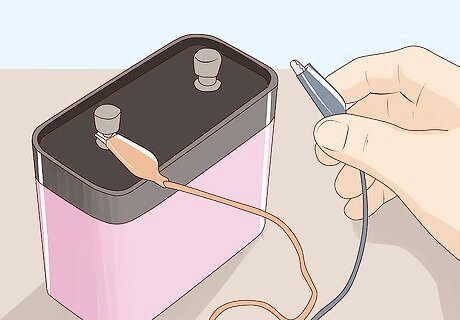
Connect one end of each alligator clip to the terminals of the battery. The battery is what actually produces the electrical current and so the alligator clips provide the pathway for transporting that current to the water. You need to attach one clip to the positive terminal and one to the negative terminal. Use a 6-volt battery but if you cannot find one, use a 9-volt battery. You can find this size of battery at pretty much any convenience store or supermarket.

Connect the other end of each alligator clip to each pencil. Make sure that the metallic part of the alligator clip is connected to the graphite of the pencil. You may have to shave down a little bit more of the wood from the pencil just to make sure that the alligator clips are completely connected to the graphite. Doing this completes the connection to the battery and allows the current to be transferred from the battery all the way through into the water.
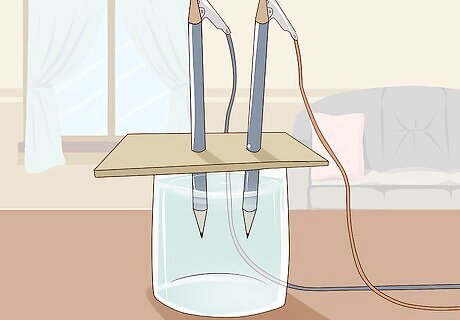
Place the cardboard on top of the glass so the pencils are submerged. The way you cut the cardboard earlier means that it should sit nicely on top of the glass. Try to do this gently so that the pencils that are stuck through the cardboard don’t get disturbed from their position. For this experiment to work, the graphite of the pencils needs to not be touching the side of the glass so just double check that here and adjust the pencils if need be.
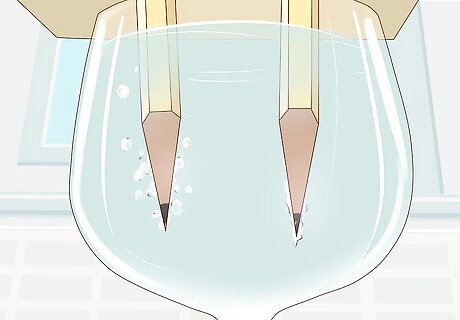
Watch the separation of hydrogen and oxygen occur. At this point, bubbles start to rise from the submerged points of graphite. This is the hydrogen and oxygen gas being split. Hydrogen gas will be bubbling from the pencil connected to the negative terminal and oxygen will be bubbling from the pencil connected to the positive terminal. Once you connect the alligator clips to the battery and graphite, the current begins to flow immediately. There will be more bubbles coming from the hydrogen pencil because there is twice as much hydrogen as oxygen in each water molecule.














Comments
0 comment