views
Extracting Citric Acid from Lemons

Wear safety goggles and gloves throughout the process. Sulfuric acid can burn your skin, irritate your eyes, and cause severe damage if enough comes into contact with you. You can wash sulfuric acid away, but it will still burn for a short time. If the burn breaks the skin and looks severe, stop what you are doing and immediately go to the hospital after flushing the area with plenty of water.
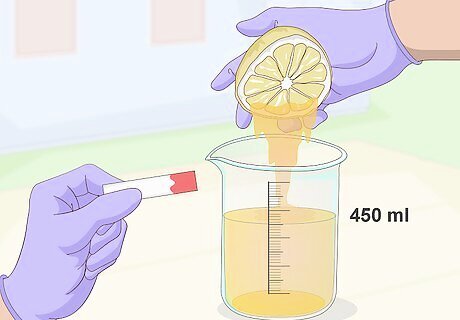
Pour 450 millilitres (1.9 c) of lemon juice into a beaker and test its pH. Lemon juice or lime juice are optimal as these fruits have a very high concentration of citric acid, making the extraction process easier. Use a pH strip to test the juice - it should be at about 2 or 3 on the pH scale. Don't use oranges, grapefruits, or other mild citrus fruits that don't immediately taste tart. These are lower in citric acid content and your end result won't be nearly as strong or effective.

Add an eyedropper filled with 10% strength sodium hydroxide and test it again. The sodium hydroxide will neutralize the acidity of the lemon juice. Add a filled eyedropper of sodium hydroxide, test the acidity with your pH testing strips, and it if it is not at about an 8 or a 9 on the pH scale, add a few more drops and test again. The solution should have a deep orange color. If you can't find 10% strength sodium hydroxide at your grocery store or chemical store, combine 10 millilitres (0.042 c) of full-strength sodium hydroxide with 90 millilitres (0.38 c) of water to make the chemical a lower strength.

Pour the solution through a coffee filter into another glass beaker. The coffee filter will separate the liquid from any solids that were produced by the reaction. If the coffee filter paper gets clogged, empty the liquid into a beaker, replace the coffee filter, and continue to pour the solution through the filter. It may take several attempts to fully filter the liquid.

Transfer the filtered solution to a new beaker and check for solids. In a clean beaker, check your liquid solution to see if it is noticeably cloudy or has solids floating in it. If there are, continue to filter it through a coffee filter until the solution is clear.

Add 28.5 g (1.01 oz) of calcium chloride to 70 ml (0.30 c) of distilled water. Do this in a separate beaker from your lemon juice solution. Mix the two together in a small beaker, and stir it until all of the calcium chloride has been dissolved.
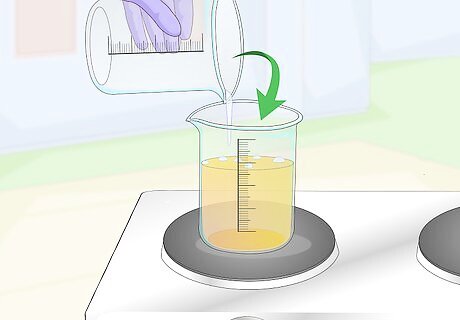
Combine both solutions and bring the mixture to a boil. Pour the calcium chloride solution into the lemon juice solution and mix it thoroughly before getting a hot plate ready. Place the beaker on the hot plate and allow the solution to come to a boil. Don't stir the solution until it boils, after which you should stir it slowly but continuously for a few minutes. Your solution will separate quickly after taking it off of the heat.
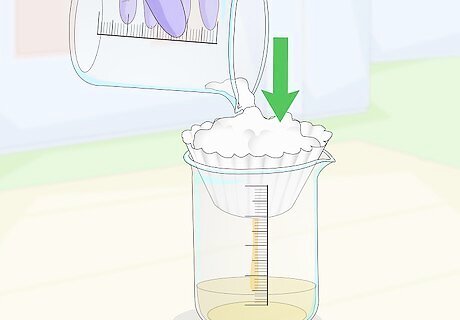
Filter the boiled solution through a coffee filter to separate the calcium citrate. The solid that formed during boiling is calcium citrate, and it should be kept separate from the liquid waste. Again, this will take a few attempts to fully filter the entire beaker. The liquid that is filtered can be discarded, but keep the calcium citrate.

Combine the calcium citrate with heavily diluted sulfuric acid and stir. Use about enough sulfuric acid to just cover the top of the calcium citrate, and stir rapidly. The exact amount you use will differ slightly based upon how much calcium citrate you produced. It won't dissolve easily, but you will end up with a pure white solution. Be extremely careful with sulfuric acid, even when it is diluted. If you get sulfuric acid on your skin, immediately stop what you are doing and flush the area with soapy water. It will irritate the acid, but it is much better to wash it off as best you can than leave it to burn your skin. For severe burns, rinse the area with plenty of water as best as you can and go straight to the hospital.
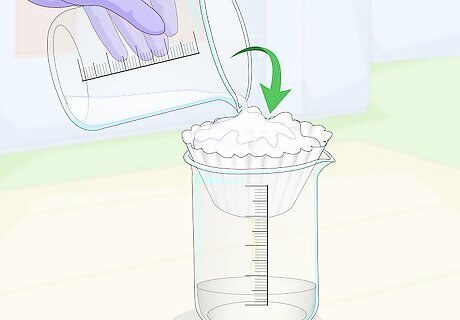
Filter the solution with water, forcing the citric acid through into a beaker. The calcium citrate has mostly converted to citric acid at this point, but it must be filtered from any impurities. The solution will be thick, so pour distilled water into the solution to help force the citric acid through. This will result in a clear liquid in your beaker containing nothing but distilled water and citric acid.
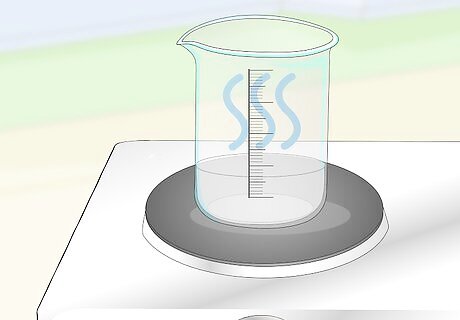
Heat this solution on medium heat to evaporate the water from the beaker. Stir the solution regularly while it is heating up, but don't allow it to boil. As the volume of the solution goes down, you will see it start to turn opaque. Wait until the volume goes down to about 70 millilitres (0.30 c), then take it off the heat.

Filter the citric acid solution to get rid of any solids then let it cool in a bowl. Using a coffee filter, pour this opaque solution through a filter into a glass bowl. The liquid filtered out will be near-pure citric acid. You can let the solution cool for longer to make a more concentrated form of citric acid. If you want to make citric acid crystals, allow the solution to sit out and evaporate for about 1 to 2 weeks. You will see the crystals start to form over time, but be careful not to disturb it. You can crush these crystals to make powder.
Making Citric Acid Solution

Buy some citric acid crystals from a grocery or chemical store. You should buy 1 pound (0.45 kg) of citric acid crystals for your solution, or more if you want it to have a higher concentration. Citric acid crystals are available at many groceries, but it is sometimes called sour salt instead of its scientific name. You can also buy it at chemical stores.
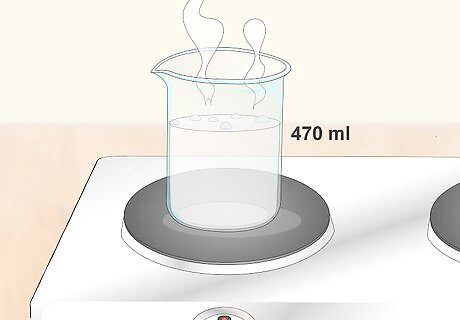
Boil 1 US pint (470 ml) of distilled water for each 1 pound (0.45 kg) of citric acid. You can use more or less water than this if you need to make the solution to be of a lower or higher concentration, but don't alter the amount of citric acid you use. For a stronger solution, boil 0.5 US pints (240 ml) to 0.75 US pints (350 ml) of distilled water per 1 pound (0.45 kg) of citric acid. This will be better for cleaning and high-intensity uses for the acid. For a weaker solution, boil up to 2 US pints (950 ml) of distilled water per 1 pound (0.45 kg) of citric acid. This can be better for adding to drinks and foods so the flavor is not overpoweringly sour.

Put your crystals into a glass container, then pour water over them. Slowly but steadily mix the boiling water and citric acid crystals, mixing with a spoon the whole time. Do this too quickly, and you will have a hard time making sure the crystals fully dissolve. Make sure to use a steady stirring motion to ensure that as much citric acid crystals dissolve as possible.
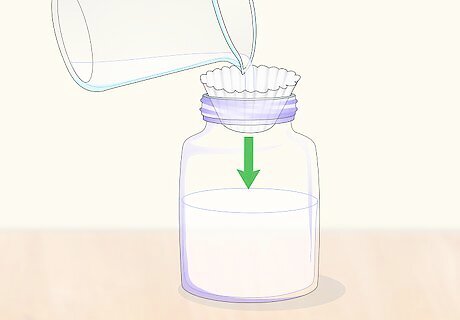
Pour the solution through a coffee filter to remove impurities and solids. Some citric acid crystals may not fully dissolve in the boiling water, and should be removed by placing a coffee filter into a funnel and pouring the solution into a glass container. Discard the undissolved crystals into an acid-proof container.
Put the solution into an acid-proof container with a lid, then refrigerate it. Refrigerate it as long as it takes it to get cool, and you will have a source of citric acid solution for use around the home or in your cooking!














Comments
0 comment