
views
Using a Battery to Charge a Battery

Remove the battery from the device. You will need access to the connection points on the battery. Keep in mind that the battery is not intended to be accessed on certain models of cell phone, so know what can be done with the model you have. On most (but not all) Android and Windows phones the back can be removed with the appropriate amount of pressure in just the right spot. Do not attempt this with most Apple products.

Find some AA, AAA, or 9-volt batteries. Unlike the power that comes from the wall outlet (alternating current), the power in common household batteries is no different from that used by your cell phone or camera battery. Perhaps you are baffled that anyone would suggest using one battery to charge another. Maybe you were expecting some magic trick that would allow you to add charge to your battery without having to find an alternate source of electric power. In fact, that is not possible. One of the fundamental laws of physics (the law of conservation of energy/conservation of mass) makes clear that you can't get something for nothing. Deal with it. It is recommended that you charge your battery rather than attempt to hotwire your electronic device and use the alternative batteries directly. Using the improper amount of amperage or voltage can potentially damage complex circuitry, so such methods are obviously not worth the risk.

Identify the positive and negative connectors on each battery. On the AAs and other household batteries, these will be marked. For most cell phone batteries, the positive connector will be the one closest to the edge, while the negative connector will usually be the one farthest from the edge (there may be three or four connectors, but the middle one or two are used for temperature regulation and other functions).

Match voltage of your battery which need to charge and other battery (AA, AAA or others with enough power to provide). Normally now a day's Cellphone battery needs more than 3.7V DC to get charged. So multiple AA or AAAs or one 9V battery will be idle for providing a charge. Keep in mind that normal AA or AAA battery on everyday household use provide 1.5V each. So to gain more than 3.7V, you need 3 AA or AAA battery connected in series. 1.5V + 1.5V + 1.5V = 4.5V would be your power source if battery on your hand is either AA or AAA model.
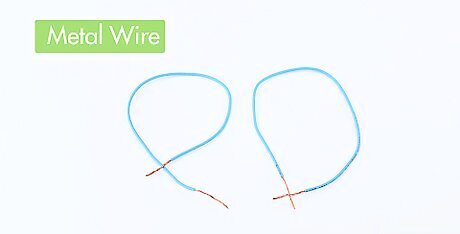
Obtain two pieces of metal wire. Ideally, these will be covered in plastic insulation except for exposed ends.
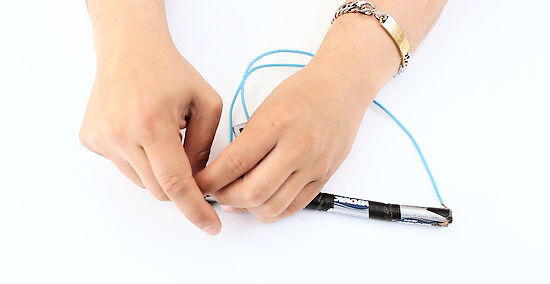
Tape or clamp the wires to the battery that will be providing a charge and the battery that requires a charge. These wires may get hot (though most likely they will not if you are doing it properly). It will also take quite a long time to transfer the charge. You don't want to be holding them the whole time. If you are using AA and AAA batteries; you may want to connect them to each other "in serie" before attaching them to the battery requiring a charge. This means using wire to connect the negative side of all the small batteries to the negative connector on the battery that needs a charge, and the same for the positive side.

After some time, the battery should be charged. Keep in mind that it probably won't be fully charged, but you should have at least some use of the device that you needed.
Using the Rubbing Trick

Remove the battery from the electronic device. Hold it in your hands.
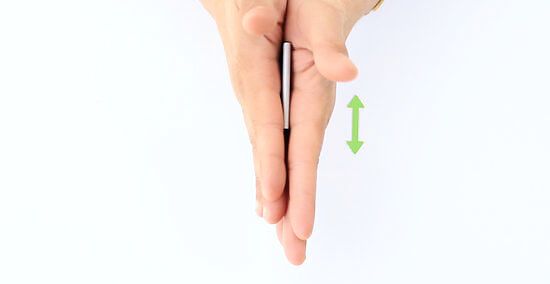
Rub the battery hard by using both of your hands to generate enough friction and heat. Continue to do this for 30 seconds to several minutes. Note: Your battery is not being recharged. Some internet commentators have suggested the rubbing the battery provides it with additional charge, perhaps from built up static electricity. This interpretation is entirely incorrect. Lithium ion cells, like all genuine batteries, release electricity as a consequence of chemical reactions. As predicted by the Arrhenius equation, these reactions become more powerful as temperatures increase. Essentially, you are improving the conductivity of the battery by raising its temperature.

Place the battery back in the electronic device. You may only have a few moments of battery life, so make the most of them.



















Comments
0 comment